Abstract
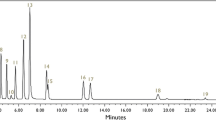
A semi-automatic two dimensional high-performance liquid chromatography (HPLC) gradient method with photo diode array detection was developed, capable of separating and quantifying up to 17 different triterpenic acids in the gum resin of the frankincense species Boswellia papyrifera. The here reported quantitation of 14 of the possible 17 compounds contains boswellic, tirucallic and lupeolic acids. All compounds were isolated from B. papyrifera and used as external standards. Quantitation of these compounds was performed after minimizing the matrix by liquid–liquid separation, using alkaline, acidic and organic media to separate the acids from interfering matrix compounds. Therefore, two different extraction procedures were tested, giving two different extraction profiles. Within the first run (1st dimension) 13 compounds could be quantified. Quantitation of β-boswellic acid, which was proved to elute as inhomogeneous peak, was achieved by introduction of a second dimension, leading to a fully validated semi-automatic homomodal 2D chromatography. The method is applicable for determination of compounds occurring in different types of frankincense and their pharmaceutical products. It also can be applied to distinguish between different kinds of frankincense. Moreover, it is the first published method feasible of separating and quantifying five different types of tirucallic acids.






Similar content being viewed by others
References
Thulin M, Warfa AM (1987) Kew Bull 42:487–493
Martinetz D, Lohs K, Janzen J (1989) Weihrauch und Myrrhe, Wissenschaftliche Verlagsgesellschaft, Stuttgart
Martinetz D (1992) Z Phytother 13:121–125
Tücker AO (1986) Econ Bot 40:425–433
Ammon HP, Mack T, Singh GB, Safayhi H (1991) Planta Med 57:203–207
Ammon HP, Safayhi H, Mack T, Sabieraj J (1993) J Ethnopharm 38:113–119
Poeckel D, Werz O (2006) Curr Med Chem 13:3359–3369
Camarda L, Dayton T, Di Stefano V, Pitonzo R, Schillaci D (2007) Ann Chim 97:837–844
Syrovets T, Büchele B, Gedig E, Slupsky JR, Simmet T (2000) Mol Pharmacol 58:71
Hoernlein RF, Orlikowski T, Simmet T, Ammon HP (1998) J Pharmacol Exp Ther 288:613
Sander O, Herborn G, Rau R (1998) Zeitschr Rheumatol 57:11–16
Kimmatkar N, Thawani V, Hingorani L, Khiyani R (2003) Phytomedicine 10:3–7
Reichling J, Schmökel H, Fitzi J, Bucher S, Saller R (2004) Schweiz Arch Tierheilkd 146:71–79
Gerhardt H, Seifert F, Buvari P, Vogelsang H, Repges R (2001) Z Gastroenterol 39:11–17
Büchele B, Zugmaier W, Simmet T (2003) J Chromatogr B 791:21–30
Ganzera M, Khan AI (2001) Planta Med 67:778–780
Shah AS, Rathod IS, Suhagia BN, Pandya SS, Parmar VK (2008) J Chromatogr Sci 46:735
Boden SE, Schweizer S, Bertsche T, Dufer M, Drews G, Safayhi H (2001) Mol Pharmacol 60:267–273
Aydee CE, Syrovets T, Pitterle K, Lunov O, Büchele B, Schimana-Pfeifer J, Schmidt T, Morad AFS, Simmet T (2010) Mol Pharmacol 77:378–387
Mondello L, Lewis AC, Bartle KD (2002) Multidimensional chromatography. John Wiley & Sons Ltd, New York
Obermann H (1977) Dragoco Rep 24:260–265
Seitz S (2008) Dissertation, Saarland University, Saarbrücken, Germany
Kromidas S, Kuss H (2008) Chromatogramme richtig integrieren und bewerten. Wiley-VCH, Weinheim
Bevington PR (1969) Data reduction and error analysis for the physical sciences. McGraw-Hill, New York
Sachs L, Hedderich J (2009) Angewandte Statistik. Springer, Berlin
Ebel S (1986) Chromatographia 22:373–378
EUROPEAN PHARMACOPOEIA 7.0, Indian Frankincense, Olibanum indicum, 1152
Schweizer S, von Brocke AFW, Boden SE, Bayer E, Ammon HP, Safayhi H (2000) J Nat Prod 63:1058–1061
Büchele B, Simmet T (2003) J Chromatrogr B 795:355–362
Sterk V, Büchele B, Simmet T (2004) Planta Med 70:1155
Abdel Tawab M, Kaunzinger A, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M (2001) J Chromatogr B 761:221–227
Mathe C, Culioli G, Archier P, Vieillescazes C (2004) Chromatographia 60:493–499
Pozharitskaya ON, Ivanova SA, Shikov AN, Makarov VG (2006) J Sep Sci 29:2245–2250
Shah AS, Rathod IS, Suhagia B, Patel DA, Parmar VK, Shah BK, Vaishnavi VM (2007) J Chromatrogr B 848:232–238
Krohn K, Rao MS, Raman NV, Khalilullah M (2001) Phytochem Anal 12:374–378
Ganzera M, Stöggl WM, Bonn GK, Khan IA, Stuppner H (2003) J Sep Sci 26:1383–1388
Reising K, Meins J, Bastian B, Eckert G, Müller WE, Schubert-Zsilavecz M, Abdel-Tawab M (2005) Anal Chem 77:6640–6645
Frank A, Unger M (2006) J Chromatorgr A 1112:255–262
Hamm S, Bleton J, Connan J, Tchapla J (2005) Phytochemistry 66:1499–1514
Hamm S, Lesellier E, Bleton J, Tchapla A (2003) J Chromatogr A 1018:73–83
Mathe C, Culioli G, Archier P, Vieillescazes C (2004) J Chromatogr A 1023:277–285
Banno N, Akihisa T, Yasukawa K, Tokuda H, Tabata K, Nakamura Y, Nishimura R, Kimura Y, Suzuki T (2006) J Ethnopharm 107:249–253
Pardhy RS, Bhattacharyya SC (1978) Indian J Chem 16B:174–175
Cotterrell GP, Halsall TG (1970) J Chem Soc C 5:739–743
Sawadogo M, Vidal-Tessier A (1985) An Ph France 43:89–96
Marques D, Graebner I, Gomes de Lemos T, Machado L, Assunção J, Queiroz Monte F (2010) Nat Prod Comm 5:1181–1182
Berendsen GE, De Galan L (1980) J Chromatogr 196:21–37
Wolfender J (2010) Potential of UHPLC for crude plant Extract analysis. In: 58th international congress and annual meeting of the society for medicinal plant and natural product research (presymposium), 29th August–2nd September 2010, Berlin, Germany. University of Geneva, Switzerland
Acknowledgments
We acknowledge the generous support of our work from Saarland University, Bundesministerium für Wirtschaft und Technologie (ProInno-Project II) and the AureliaSan GmbH.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paul, M., Brüning, G., Weihrather, J. et al. Qualitative and Quantitative Analysis of 17 Different Types of Tetra- and Pentacyclic Triterpenic Acids in Boswellia papyrifera by a Semi-Automatic Homomodal 2D HPLC method. Chromatographia 74, 29–40 (2011). https://doi.org/10.1007/s10337-011-2041-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-011-2041-3