Abstract
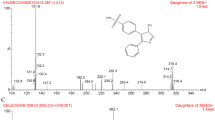
Oroxylin A shows reversal activity on multidrug resistance and has an attractive safety profile. A highly sensitive and specific method based on liquid chromatography coupled with tandem mass spectroscopy was developed and validated for simultaneous determination of oroxylin A and its major metabolite, oroxylin A-7-O-glucuronide in rat plasma. The assay procedure involved precipitation of plasma samples with acetonitrile after adding luteolin as an internal standard. Chromatographic separation was achieved on a C18 column using methanol and 0.5% formic acid as the mobile phase, eluting in a gradient system. Detection was achieved with selected reaction monitoring using the electrospray ionization technique. The method was validated by evaluation of selectivity, linearity, precision, accuracy and limit of quantification. Its applicability was demonstrated through the quantification of oroxylin A and oroxylin A-7-O-glucuronide in plasma after intravenous administration of oroxylin A at a dose of 20 mg kg−1 to rats. The developed quantification method can now be used for pharmacokinetic studies after intravenous infusion of oroxylin A injection in rats.



Similar content being viewed by others
References
Hu Y, Yang Y, You QD, Liu W, Gu HY, Zhao L, Zhang K, Wang W, Wang XT, Guo QL (2006) Biochem Biophys Res Commun 351:521–527
Li HN, Nie FF, Liu W, Dai QS, Lu N, Qi Q, Li ZY, You QD, Guo QL (2009) Toxicology 257:80–85
Sun Y, Lu N, Ling Y, Gao Y, Chen Y, Wang L, Hu R, Qi Q, Liu W, Yang Y, You Q, Guo Q (2009) Eur J Pharmacol 603:22–28
Yang LX, Liu D, Feng XF, Cui SL, Yang JY, Tang XJ, He XR, Liu JF, Hu SL (2002) China J Chin Mater Med 27:166–170
Zuo F, Zhou ZM, Zhang Q, Mao D, Xiong YL, Wang YL, Yan MZ, Liu ML (2003) Biol Pharm Bull 26:911–919
Kim YH, Jeong DW, Paek IB, Ji HY, Kim YC, Sohn DH, Lee HS (2006) J Chromatogr B Anal Technol Biomed Life Sci 844:261–267
FDA (1994) Food and Drug Administration, Center of Drug Evolution and Research, 1994, Reviewer guidance, validation of chromatographic methods
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, W., Xu, X., Feng, F. et al. Simultaneous Quantification of Oroxylin A and Its Metabolite Oroxylin A-7-O-Glucuronide: Application to a Pharmacokinetic Study in Rat. Chromatographia 74, 75–81 (2011). https://doi.org/10.1007/s10337-011-2020-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-011-2020-8