Abstract
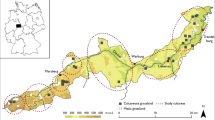
The endangered continental Black-tailed Godwit (Limosa limosa limosa) is a migratory ground-nesting wader breeding in a wide variety of open, wet habitats across Europe. Conservation research has concentrated on the causes of population decline, but we know surprisingly little about whether any resources limit local breeding populations and if so, whether these are resources for the adults or the chicks. We collected data from 63 key breeding sites in five countries across Europe to test whether, after correcting for differences in surveyed areas, the size of Godwit breeding populations was related to environmental variables (vegetation biomass, soil moisture) or food resources for adult birds (soil invertebrates) or chicks (vegetation dwelling arthropods) measured during different times of the reproductive cycle. We found the number of Godwit territories to be positively related to arthropod abundance during the chick-hatching period. We found additional, weaker support for a positive relation between Godwit territory numbers and the abundance of soil-dwelling invertebrates (mostly earthworms) at clutch laying, but not at chick-hatching. These relationships were observed across countries, while we found little support for relationships within countries, possibly due to the smaller range in conditions that exist within countries. Both vegetation growth and soil moisture weren’t related to Godwit territory numbers. Our results suggest that food abundance for chicks, and to a lesser extent adult birds, are key factors determining the size of local Godwit breeding populations. Conservation management aiming to enhance local Godwit populations should therefore consider the impacts of management strategies on the arthropod prey of chicks.
Zusammenfassung
Kontinentale Unterschiede in den Brutdichten der Uferschnepfe lassen sich am besten durch das Vorkommen von Arthropoden in der Zeit des Schlüpfens der Küken erklären.
Die Uferschnepfe (Limosa limosa limosa) ist ein ziehender, bodenbrütender Watvogel, der in einer Vielzahl von offenen, feuchten Lebensräumen in ganz Europa brütet. Die Naturschutzforschung hat sich auf die Ursachen des Populationsrückgangs konzentriert, aber wir wissen erstaunlich wenig darüber, ob es irgendwelche Ressourcen gibt, die lokale Brutpopulationen begrenzen und wenn ja, ob es sich dabei um Ressourcen für Altvögel oder Küken handelt. Wir haben Daten von 63 großen Brutplätzen in fünf europäischen Ländern gesammelt, um zu prüfen, ob - nach Ausgleichen der Unterschiede in den untersuchten Gebieten - die Größe der Uferschnepfen-Brutpopulationen mit Umweltfaktoren (Biomasse der Vegetation, Bodenfeuchtigkeit) oder Nahrungsressourcen für Altvögel (im Boden lebende Wirbellose) oder Küken (in der Vegetation lebende Arthropoden), deren Mengen zu verschiedenen Zeiten des Fortpflanzungszyklus erfasst wurden, zusammenhängt. Wir stellten fest, dass die Anzahl der Uferschnepfenreviere positiv mit dem Arthropodenaufkommen während der Schlüpfzeit der Küken zusammenhängt. Ferner fanden wir weitere, schwächere Belege für eine positive Korrelation zwischen der Anzahl der Uferschnepfenreviere und dem Vorkommen von bodenbewohnenden Wirbellosen (hauptsächlich Regenwürmern) zur Zeit der Eiablage, aber nicht des Schlüpfens der Küken. Diese Zusammenhänge wurden länderübergreifend beobachtet, während wir innerhalb der Länder kaum Belege für Zusammenhänge fanden, was möglicherweise auf die geringere Variationsbreite der Bedingungen innerhalb der Länder zurückzuführen ist. Weder die Vegetationsentwicklugn noch die Bodenfeuchte zeigten einen Zusammenhang mit der Anzahl der Uferschnepfenreviere. Unsere Ergebnisse deuten darauf hin, dass das Nahrungsangebot für die Küken und in geringerem Maße für die Altvögel ein Schlüsselfaktor für die Größe der örtlichen Uferschnepfenpopulationen ist. Schutzmaßnahmen zum Erhalt des Bestands und zur Stärkung lokaler Uferschnepfenpopulationen sollten deshalb die Auswirkungen von Managmentmaßnahmen auf die Arthropoden-Beute der Küken berücksichtigen.



Similar content being viewed by others
Data availability
Data will be available via the Dryad Digital Repository.
References
Andrey A, Humbert J, Pernollet C, Arlettaz R (2014) Experimental evidence for the immediate impact of fertilization and irrigation upon the plant and invertebrate communities of mountain grasslands. Ecol Evol 4:2610–2623. https://doi.org/10.1002/ece3.1118
Babyak MA (2004) What you see may not be what you get: A brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med 66:411–421. https://doi.org/10.1097/01.psy.0000127692.23278.a9
Bakker W, Ens BJ, Dokter A et al (2021) Connecting foraging and roosting areas reveals how food stocks explain shorebird numbers. Estuar Coast Shelf Sci 259:107458. https://doi.org/10.1016/J.ECSS.2021.107458
Beintema AJ, Visser GH (1989) The effect of weather on time budgets and development of chicks of meadow birds. Ardea 77:181–192
Beintema AJ, Thissen JB, Tensen D, Visser GH (1991) Feeding ecology of charadriiform chicks in agricultural grassland. Ardea 79:31–44
Bellebaum J, Bock C (2008) Influence of ground predators and water levels on Lapwing Vanellus vanellus breeding success in two continental wetlands. J Ornithol 150:221–230. https://doi.org/10.1007/S10336-008-0341-7
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav Ecol Sociobiol 65:23–35
De Felici L, Piersma T, Howison RA (2019) Abundance of arthropods as food for meadow bird chicks in response to short- And long-term soil wetting in Dutch dairy grasslands. PeerJ. https://doi.org/10.7717/peerj.7401
Eglington SM, Bolton M, Smart MA et al (2010) Managing water levels on wet grasslands to improve foraging conditions for breeding northern lapwing Vanellus vanellus. J Appl Ecol 47:451–458. https://doi.org/10.1111/j.1365-2664.2010.01783.x
Fraixedas S, Lindén A, Meller K et al (2017) Substantial decline of Northern European peatland bird populations: Consequences of drainage. Biol Conserv 214:223–232. https://doi.org/10.1016/j.biocon.2017.08.025
Gill JA, Langston RHW, Alves JA et al (2007) Contrasting trends in two Black-tailed Godwit populations: a review of causes and recommendations. Wader Study Gr Bull 114:43–50
Goss-Custard JD (1991) Towards predicting wading bird densities from predicted prey densities in a post-barrage Severn Estuary. J Appl Ecol 28:1004–1026. https://doi.org/10.2307/2404222
Graham MH (2003) Confronting multicollinearity in ecological multiple regression. Ecology 84:2809–2815. https://doi.org/10.1890/02-3114
Groen NM, Kentie R, de Goeij P et al (2012) A modern landscape ecology of black-tailed godwits: habitat selection in southwest friesland, The Netherlands. Ardea 100:19–28. https://doi.org/10.5253/078.100.0105
Haddad NM, Haarstad J, Tilman D (2000) The effects of long-term nitrogen loading grassland insect communities. Oecologia 124:73–84. https://doi.org/10.1007/s004420050026
Jensen FP, Perennou C (2007) Management plan for Black-tailed Godwit (Limosa limosa) 2007–2009. Office for Official Publications of the European Communities, Luxembourg
Kamp J, Reinhard A, Frenzel M et al (2018) Farmland bird responses to land abandonment in Western Siberia. Agric Ecosyst Environ 268:61–69. https://doi.org/10.1016/j.agee.2018.09.009
Kentie R, Hooijmeijer JCEW, Trimbos KB et al (2013) Intensified agricultural use of grasslands reduces growth and survival of precocial shorebird chicks. J Appl Ecol 50:243–251. https://doi.org/10.1111/1365-2664.12028
Kentie R, Both C, Hooijmeijer JCEW, Piersma T (2014) Age-dependent dispersal and habitat choice in black-tailed godwits Limosa limosa limosa across a mosaic of traditional and modern grassland habitats. J Avian Biol 45:396–405. https://doi.org/10.1111/jav.00273
Kentie R, Both C, Hooijmeijer JCEW, Piersma T (2015) Management of modern agricultural landscapes increases nest predation rates in Black-tailed Godwits Limosa limosa. Ibis 157:614–625. https://doi.org/10.1111/ibi.12273
Kentie R, Senner NR, Hooijmeijer JCEW et al (2016) Estimating the Size of the Dutch Breeding Population of Continental Black-Tailed Godwits from 2007–2015 Using Resighting Data from Spring Staging Sites. Ardea 104:213–225. https://doi.org/10.5253/arde.v104i3.a7
Kentie R, Coulson T, Hooijmeijer JCEW et al (2018) Warming springs and habitat alteration interact to impact timing of breeding and population dynamics in a migratory bird. Glob Chang Biol 24:5292–5303. https://doi.org/10.1111/gcb.14406
Kleijn D, Dimmers WJ, van Kats RJM, Melman TCP (2009a) Het belang van hoog waterpeil en bemesting voor de Grutto: I. de vestigingsfase. Levende Nat 110:180–183
Kleijn D, Schekkerman H, Dimmers WJ et al (2010) Adverse effects of agricultural intensification and climate change on breeding habitat quality of Black-tailed Godwits Limosa l. limosa in the Netherlands. Ibis 152:475–486. https://doi.org/10.1111/j.1474-919X.2010.01025.x
Kruk M, Noordervliet MAW, Keurs WJT (1998) Natal philopatry in the Black-tailed Godwit Limosa limosa L. And its possible implications for conservation. Ringing Migr 19:13–16. https://doi.org/10.1080/03078698.1998.9674156
Laidlaw RA, Smart J, Smart MA, Gill JA (2017) Scenarios of habitat management options to reduce predator impacts on nesting waders. J Appl Ecol 54:1219–1229. https://doi.org/10.1111/1365-2664.12838
Lebedeva EA (1998) Waders in agricultural habitats of European Russia. Int Wader Stud 10:315–324
Leito A, Elts J, Mägi E et al (2014) Coastal grassland wader abundance in relation to breeding habitat characteristics in Matsalu Bay, Estonia. Ornis Fenn 91:149–165
Loonstra AHJ, Verhoeven MA, Senner NR et al (2019) Natal habitat and sex-specific survival rates result in a male-biased adult sex ratio. Behav Ecol 30:843–851. https://doi.org/10.1093/beheco/arz021
Onrust J, Wymenga E, Piersma T, Olff H (2019) Earthworm activity and availability for meadow birds is restricted in intensively managed grasslands. J Appl Ecol 56:1333–1342. https://doi.org/10.1111/1365-2664.13356
Quinn GP, Keough MJ (2002) Experimental Design and Data Analysis for Biologists. Exp Des Data Anal Biol. https://doi.org/10.1017/CBO9780511806384
Roodbergen M, van der Werf B, Hötker H (2012) Revealing the contributions of reproduction and survival to the Europe-wide decline in meadow birds: review and meta-analysis. J Ornithol 153:53–74. https://doi.org/10.1007/s10336-011-0733-y
Schekkerman H, Beintema AJ (2007) Abundance of Invertebrates and Foraging Success of Black-Tailed Godwit Limosa limosa Chicks in Relation to Agricultural Grassland Management. Ardea 95:39–54. https://doi.org/10.5253/078.095.0105
Schekkerman H, Teunissen W, Oosterveld E (2009) Mortality of Black-tailed Godwit Limosa limosa and Northern Lapwing Vanellus vanellus chicks in wet grasslands: influence of predation and agriculture. J Ornithol 150:133–145
Silva-Monteiro M, Pehlak H, Fokker C et al (2021) Habitats supporting wader communities in Europe and relations between agricultural land use and breeding densities: A review. Glob Ecol Conserv 28:e01657. https://doi.org/10.1016/j.gecco.2021.e01657
Silva-Monteiro M, Scheper J, Pehlak H et al (2022) Invertebrate abundance increases with vegetation productivity across natural and agricultural wader breeding habitats in Europe. Biol Conserv 273:109670. https://doi.org/10.1016/j.biocon.2022.109670
Struwe-Juhl B (1995) Auswirkungen der Renaturierungsmaßnahmen im Hohner See-Gebiet auf Bestand, Bruterfolg und Nahrungsökologie der Uferschnepfe (Limosa limosa). Corax 16:153–172
Symonds MRE, Moussalli A (2011) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav Ecol Sociobiol 65:13–21
Thorup O (2006) Breeding waders in Europe 2000. International Wader Studies. https://doi.org/10.5253/078.100.0105
van de Pol M, Wright J (2009) A simple method for distinguishing within- versus between-subject effects using mixed models. Anim Behav 77:753–758
van Paassen AG, Veldman DH, Beintema AJ (1984) A simple device for determination of incubation stages in eggs. Wildfowl 35:173–178
Verhoeven MA, Loonstra AHJ, McBride AD et al (2020) Geolocators lead to better measures of timing and renesting in Black-tailed Godwits and reveal the bias of traditional observational methods. J Avian Biol. https://doi.org/10.1111/jav.02259
Zharikov Y, Skilleter GA (2003) Depletion of benthic invertebrates by bar-tailed godwits Limosa lapponica in a subtropical estuary. Mar Ecol Prog Ser 254:151–162. https://doi.org/10.3354/MEPS254151
Żmihorski M, Krupiński D, Kotowska D et al (2018) Habitat characteristics associated with occupancy of declining waders in Polish wet grasslands. Agric Ecosyst Environ 251:236–243. https://doi.org/10.1016/j.agee.2017.09.033
AEWA (2008) International Single Species Action Plan for the Conservation of the Black-tailed Godwit (TS No. 37) | AEWA. Agreement on the Conservation of African-Eurasian Migratory Waterbirds
Barton K (2020) MuMIn: Multi-Model Inference. R Package version 1.43.17. https://cran.r-project.org/web/packages/MuMIn/index.html. Accessed 8 Feb 2021
Beintema AJ (Albert J, Moedt O, Ellinger D (1995) Ecologische atlas van de Nederlandse weidevogels. Schuyt
Bibby C, Burgess N, Hill D (1992) Bird Census Techniques - 1st Edition
Bijlsma RG, Hustings F, Camphuysen CJ (2001) Algemene en schaarse vogels van Nederland. Avifauna van Nederland, 2.
Brooks ME, Kristensen K, van Benthem KJ, et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9: 378–400. https://doi.org/10.32614/rj-2017-066
Haverschmidt F (1963) The black‐tailed godwit. E J Brill, Leiden
Keller V, Herrando S, Voříšek P, et al (2021) European Breeding Bird Atlas 2: Distribution, Abundance and Change. European Bird Census Council & Lynx Edicions, Barcelona
Kleijn D, Dimmers WJ, Kats RJM van, Melman TCP (2009b) De relatie tussen gebruiksintensiteit en de kwaliteit van graslanden als foerageerhabitat voor gruttokuikens. Alterra.
Kleijn D, Lammertsma D, Müskens G (2011) Het belang van waterpeil en bemesting voor de voedselbeschikbaarheid van weidevogels pp. 41–60. SOVON onderzoeksrapport 2011/10
Ławicki Ł, Kruszyk R (2011) Long-term decline of the grassland waders in Western Poland, VOGELWELT.
Mulder T (1972) De Grutto (Limosa limosa (L.)) in Nederland: aantallen, verspreiding, terreinkeuze, trek en overwintering
Piersma T (2012) What is habitat quality? Dissecting a research portfolio on shorebirds. In: Birds and Habitat. Cambridge University Press, pp 383–407
R Core Team (2017) R: A language and environment for statistical computing.
Strus YM, Shydlovskyy IV, Gorban IM (2018) Grassland waders in the upper Prypiat’ basin: spatial distribution and number dynamics. “Branta” Trans Azov-Black Sea Ornithol Stn 2018: 53–72. https://doi.org/10.15407/BRANTA2018.21.053
Acknowledgements
MSM was funded by a grant from Wageningen University & Research to DK (proj. nr. 5160957485). JL and MV and the research infrastructure in NL was supported by the Province of Fryslân and the NWO-Spinoza Premium 2014 to Theunis Piersma. Thanks to Kim Peterse, Ilona Lepik and Samuel Leeming for the support in the fieldwork conducted in Finland and Estonia. Thanks to Tomasz Tumiel, Paweł Białomyzy and Grzegorz Grygoruk from Nature Association Dubelt for the support in the fieldwork conducted in Poland. Thanks to Jean-Guy Robin for the support in the fieldwork conducted in France.
Author information
Authors and Affiliations
Contributions
MSM and DK conceived the ideas and designed methodology; MSM, HP, ST, JP, EP, MV, JL, FR, MK, MO, AD, MB, JH and FL collected the data; MSM analyzed the data; MSM and DK led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silva-Monteiro, M., Pehlak, H., Timonen, S. et al. Cross-continental differences in Black-tailed Godwit breeding densities are best explained by arthropod abundance in the chick-hatching period. J Ornithol 164, 287–297 (2023). https://doi.org/10.1007/s10336-022-02041-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-022-02041-9