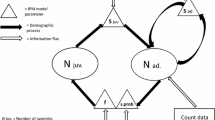
Abstract
Jankowski’s Bunting Emberiza jankowskii is one of several grassland birds that have suffered major population declines across their ranges, and the cause of these declines remains largely unknown. To determine what demographic drivers are responsible for their decline, we combined specific annual female productivity from a local Jankowski’s Bunting population and survival probabilities from the Ortolan Bunting, an ecologically similar species. We used an age-structured matrix population model to examine the population dynamics of Jankowski’s Bunting and showed that they may not be capable of sustaining a stable population, even without environmental stochasticity and density dependence. Compared to other Emberiza buntings with similar population trends, our results indicate that the population decline in Jankowski’s Bunting is largely caused by a particularly low reproductive success, and in particular a very low survival of eggs and nestlings. Despite the relatively low elasticity of the population dynamics to breeding parameters, our analysis suggests that increasing the number of fledglings to levels similar to those of closely related species would result in a growing population. Given that the reproductive success is highly influenced by nest predation or human disturbance, we suggest that initial conservation actions reducing interference from human activities are meaningful to improve the reproductive success of remaining Jankowski’s Bunting populations and allow the species to persist in the long term.
Zusammenfassung
Geringe Überlebensraten von Eiern und Nestlingen erklären den Rückgang einer lokalen Population der Jankowskiammer
Die Jankowskiammer (Emberiza jankowskii) ist eine von mehreren Wiesenvögeln, deren Bestände in ihrem gesamten Verbreitungsgebiet stark zurückgegangen sind, wobei die Ursache für diesen Rückgang noch weitgehend unbekannt ist. Um festzustellen, welche demographischen Faktoren für ihren Rückgang verantwortlich sind, haben wir spezifische Bruterfolgsparameter einer lokalen Jankowskiammer-Population mit Überlebenswahrscheinlichkeiten des Ortolans, einer ökologisch ähnlichen Art, kombiniert. Hierfür verwendeten wir ein nach Alter strukturiertes Matrix-Populationsmodell, um die Populationsdynamik der Jankowskiammer zu untersuchen und konnten zeigen, dass sie möglicherweise nicht in der Lage ist, eine stabile Population (Populationswachstumsrate λ=0,77) aufrechtzuerhalten, selbst wenn man dabei Umweltstochastik und Dichteabhängigkeit außer Acht lässt. Im Vergleich zu anderen Emberiza-Arten mit ähnlichen Populationstrends deuten unsere Ergebnisse darauf hin, dass der Populationsrückgang bei der Jankowskiammer zum größten Teil an einem besonders geringen Bruterfolg und insbesondere an einer sehr geringen Überlebensrate der Eier und Nestlinge liegt. Trotz der relativ geringen Anfälligkeit der Populationsdynamik gegenüber den Brutparametern deutet unsere Analyse darauf hin, dass eine Erhöhung der Zahl der Jungvögel auf ein ähnliches Niveau wie bei eng verwandten Arten zu einer wachsenden Population führen würde. Da der Bruterfolg in hohem Maße von Nesträubern und Störungen durch den Menschen beeinflusst wird, schlagen wir vor, dass zunächst Schutzmaßnahmen zur Verringerung der Störungen durch den Menschen vorgenommen werden sollten, um den Bruterfolg der noch vorhandenen Jankowskiammer-Populationen zu verbessern und den Fortbestand der Art langfristig zu sichern.



Similar content being viewed by others
Availability of data and materials
Not applicable.
References
Akçakaya HR, Sjögren-Gulve P (2000) Population viability analyses in conservation planning: an overview. Ecol Bull 48:9–21
Balmer D, Peach W, Norfolk I (1997) Review of natural avian mortality rates. BTO Res Rep 175:13–17
Beissinger SR, McCullough DR (2002) Population viability analysis. University of Chicago Press, Chicago
Bennett PM, Owens IPF (1997) Variation in extinction risk among birds: chance or evolutionary predisposition? Proc R Soc B 264:401–408
BirdLife International (2021) Species factsheet: Emberiza jankowskii. BirdLife International, Cambridge
Bradbury RB, Kyrkos A, Morris AJ et al (2000) Habitat associations and breeding success of yellowhammers on lowland farmland. J Appl Ecol 37:789–805
Brickle NW, Harper DGC, Aebischer NJ et al (2000) Effects of agricultural intensification on the breeding success of corn buntings Miliaria calandra. J Appl Ecol 37:742–755
Bro E, Sarrazin F, Clobert J, Reitz F (2000) Demography and the decline of the grey partridge Perdix perdix in France. J Appl Ecol 37:432–448
Brook BW, Kikkawa J (1998) Examining threats faced by island birds: a population viability analysis on the Capricorn silvereye using long-term data. J Appl Ecol 35:491–503
Choi CY, Nam HY, Kim HK et al (2020) Changes in Emberiza bunting communities and populations spanning 100 years in Korea. PLoS ONE 15(5):e0233121. https://doi.org/10.1371/journal.pone.0233121
Cooper CB, Walters JR (2002) Experimental evidence of disrupted dispersal causing decline of an Australian passerine in fragmented habitat. Conserv Biol 16:471–478
Coulson T, Mace GM, Hudson E, Possingham H (2001) The use and abuse of population viability analysis. Trends Ecol Evol 16:219–221
Crick HQ, Donald PF, Greenwood J (1991) Population processes in some British seed-eating birds. Br Trust Ornithol 80:26–28
Donald PF, Green R, Heath M (2001) Agricultural intensification and the collapse of Europe’s farmland bird populations. Proc R Soc Lond Ser B 268:25–29
Donald PF, Sanderson FJ, Burfield IJ, Van Bommel FP (2006) Further evidence of continent-wide impacts of agricultural intensification on European farmland birds, 1990–2000. Agr Ecosyst Environ 116:189–196
Edenius L, Choi C, Heim W, Jaakkonen T, De Jong A, Ozaki K, Roberge J (2017) The next common and widespread bunting to go? Global population decline in the Rustic Bunting Emberiza rustica. Bird Conser Int 27(1):35–44. https://doi.org/10.1017/S0959270916000046
Evans A (1992) The numbers and distribution of Cirl Buntings Emberiza cirlus breeding in Britain in 1989. Bird Study 39:17–22
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fu TS, Chen P (1966) The distribution and breeding habitats of Emberiza jankowskii. Acta Zool Sin 18:197–198
Gao W (2002) Ecology in Jankowski’s Bunting. Science and Technology Press, Jilin
Han Z, Zhang L, Jiang Y, Wang H, Jiguet F (2020) Unravelling species co-occurrence in a steppe bird community of Inner Mongolia: Insights for the conservation of the endangered Jankowski’s Bunting. Divers Distrib 26:843–852. https://doi.org/10.1111/ddi.13061
Han Z, Zhang LS, Qin B et al (2018) Updated breeding distribution and population status of Jankowski’s Bunting Emberiza jankowskii in China. Bird Conserv Int 28:643–652
Han Z, Wang HT, Kardynal KJ (2021) Stable isotopes (δ2H) in feathers identify non-breeding origins of the endangered Jankowski’s Bunting. J Ornithol 162:987–995. https://doi.org/10.1007/s10336-021-01897-7
Inchausti P, Halley J (2003) On the relation between temporal variability and persistance time in animal populations. J Anim Ecol 72:899–908
Jeffs C, Sainsbury A, Davies M et al (2016) Reintroducing the cirl bunting to Cornwall. Br Birds 109:374–388
Jiang YL, Gao W, Lei FM et al (2008) Nesting biology and population dynamics of Jankowski’s Bunting Emberiza jankowskii in Western Jilin, China. Bird Conserv Int 18:153–163
Jiguet F, Devictor V, Ottvall R et al (2010) Bird population trends are linearly affected by climate change along species thermal ranges. Proc R Soc B 277:3601–3608
Jiguet F, Robert A, Lorrillière R et al (2019) Unravelling migration connectivity reveals unsustainable hunting of the declining ortolan bunting. Sci Adv 5:eaau2642
Kämmerle JL, Niekrenz S, Storch I (2019) No evidence for spatial variation in predation risk following restricted-area fox culling. BMC Ecol 19:17
Kamp J, Oppel S, Ananin AA, Durnev YA, Gashev SN, Hölzel N, Mishchenko AL, Pessa J, Smirenski SM, Strelnikov EG, Timonen S, Wolanska K, Chan S (2015) Global population collapse in a superabundant migratory bird and illegal trapping in China. Conserv Biol 29:1684–1694. https://doi.org/10.1111/cobi.12537
Legendre CJ, Moller AP et al (1999) Demographic stochasticity and social mating system in the process of extinction of small populations: the case of passerines introduced to New Zealand. Am Nat 153:449–463
Macleod CJ, Parish DM, Duncan RP et al (2005) Do Yellowhammers Emberiza citrinella achieve higher breeding productivity in their introduced range than in their native range? Bird Study 52:217–220
Millon A, Lambin X, Devillard S et al (2019) Quantifying the contribution of immigration to population dynamics: a review of methods, evidence and perspectives in birds and mammals. Biol Rev 94:2049–2067
Moritz C, Agudo R (2013) The future of species under climate change: resilience or decline? Science 341:504–508
Morris WF, Doak DF (2002) Quantitative conservation biology. Sinauer, Sunderland
Murray BG (2000) Measuring annual reproductive success in birds. Condor 102(2):470–473
Muzika Y, Fu V, Townshend T et al (2015) Ornithological survey in Dornod province, eastern Mongolia. Birding Asia 24:54–63
Newton I (2004) The recent declines of farmland bird populations in Britain: an appraisal of causal factors and conservation actions. Ibis 146:579–600
Oelke H (1980) Bird census work and nature conservation. Dachverband Deutscher Avifaunisten, Münster
Parkhust K, David L (1946) The clutch-size of the yellowhammer. Notes 24:3
Peach WJ, Siriwardena GM, Gregory RD (1999) Long-term changes in over-winter survival rates explain the decline of reed buntings Emberiza schoeniclus in Britain. J Appl Ecol 36:798–811
Perkins AJ, Maggs HE, Wilson JD et al (2013) Delayed mowing increases corn bunting Emberiza calandra nest success in an agri-environment scheme trial. Agric Ecosyst Environ 181:80–89
Ponz A, Barba E, Gil-Delgado J (1996) Population changes and breeding ecology of the Cirl Bunting Emberiza cirlus in eastern Spain. Bird Study 43:38–46
Ricklefs RE (1969) An analysis of nesting mortality in birds. Smithsonian contributions to zoology. Smithson Contrib Zool 9:1–48
Rosenberg KV, Dokter AM, Blancher PJ (2019) Decline of the North American avifauna. Science 366:eaaw113
Sæther BE, Engen S, Islam A et al (1998) Environmental stochasticity and extinction risk in a population of a small songbird, the great tit. Am Nat 151:441–450
Schaub M, Ullrich B (2021) A drop in immigration results in the extinction of a local woodchat shrike population. Anim Conserv 24:335–345. https://doi.org/10.1111/acv.12639
Sillett TS, Holmes RT (2002) Variation in survivorship of a migratory songbird throughout its annual cycle. J Anim Ecol 71:296–308
Siriwardena GM, Baillie SR, Wilson JD (1998) Variation in the survival rates of some British passerines with respect to their population trends on farmland. Bird Study 45:276–292
Siriwardena GM, Baillie SR, Wilson JD (1999) Temporal variation in the annual survival rates of six granivorous birds with contrasting population trends. Ibis 141:621–636
Siriwardena GM, Baillie SR, Crick HQP et al (2000) The importance of variation in the breeding performance of seed-eating birds in determining their population trends on farmland. J Appl Ecol 37:128–148
Stanton R, Morrissey C, Clark R (2018) Analysis of trends and agricultural drivers of farmland bird declines in North America: a review. Agric Ecosyst Environ 254:244–254
Steifetten Ø, Dale S (2006) Viability of an endangered population of ortolan buntings: the effect of a skewed operational sex ratio. Biol Conserv 132:88–97
Stresemann E, Portenko LA (1981) Atlas der Verbreitung Palaearktischer Vo¨gel, 9. Akademie-Verlag, Berlin
Stubben C, Milligan B, Nantel P (2008) Popbio: construction and analysis of matrix population models. R Package Version 1.
Sulawa J, Robert A, Köppen U et al (2010) Recovery dynamics and viability of the white-tailed eagle (Haliaeetus albicilla) in Germany. Biodivers Conserv 19:97–112
Sundberg J (1995) Parasites, plumage coloration and reproductive success in the yellowhammer, Emberiza citrinella. Oikos 74:331–339
Tempel DJ, Peery M, Gutierrez RJ (2014) Using integrated population models to improve conservation monitoring: California spotted owls as a case study. Ecol Model 289:86–95
Thompson BC, Gregory EK, Donald LB et al (2001) Nest success is not an adequate comparative estimate of avian reproduction. J Field Ornithol 72(4):527–536
Tong FC, Xiao YH, Bai HS et al (2002) Breeding ecology of Jankowski’s Bunting in the dry grassland in Baicheng, Jilin Province. Acta Ecol Sincia 22:1485–1490
Wang H, Sun DT (2003) The habitat and nest-site slection of Jankowski’s bunting. Acta Ecol Sin 23:665–672
Wang H, Jiang Y, Gao W (2010) Jankowski’s Bunting (Emberiza jankowskii): current status and conservation. Chin Birds 1:251–258
Watson H, Bolton M, Monaghan P (2014) Out of sight but not out of harm’s way: Human disturbance reduces reproductive success of a cavity-nesting seabird. Biol Conserv 174(100):127–133
Yom-Tov Y (1992) Clutch size and laying dates of three species of buntings Emberiza in England. Bird Study 39:111–114
Yom-Tov Y, Mccleery R, Purchase D (1992) The survival rate of Australian passerines. Ibis 134:374–379
Acknowledgements
This research was funded by the National Nature Science Foundation of China (No. 31971402) and the China Scholarship Council (CSC). We thank Steffen Oppel and other anonymous reviewers for their helpful remarks on this paper.
Author information
Authors and Affiliations
Contributions
HW and FJ contributed equally and should be considered as co-corresponding and senior authors. H.W. and F.J. conceived the project. Z.H. and A.R. analyzed the data. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical declarations
Ethics approval and consent to participate. Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Han, Z., Robert, A., Wang, H. et al. Low survival of eggs and nestlings explain the decline of a local Jankowski’s Bunting population. J Ornithol 163, 817–826 (2022). https://doi.org/10.1007/s10336-022-01983-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-022-01983-4