Abstract
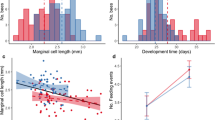
Theory predicts that organisms are apt to optimize their resource allocation strategy and since rearing offspring is costly, condition might predict individual fitness as individuals in better condition hold more resources to allocate towards maximizing their reproductive success. We use a 3 year study (29 pairs, 58 broods, 366 nestlings) of Common Kingfisher Alcedo atthis to examine how parents’ age, scaled mass index (SMI) and heterophile-to-lymphocyte ratio (H/L ratio) influence breeding success of this species. Fieldwork was carried out on the Danube River system from Bratislava to Gabčíkovo (south-western Slovakia) during 2016–2018. We observed that males and females differed in values of H/L ratio and SMI with better overall physical condition for females. At the same time, these two variables neither correlated with each other nor was there a difference between age groups. Further analyses revealed that females mated with males of lower H/L ratio tended to initiate their clutches earlier in the season. Concurrently, pairs which had laid their first brood earlier in the breeding season reared nestlings from more broods during that season. Number of broods was also significantly affected by age of female and male SMI, with older females and males in good condition more likely to produce more broods. Contrary to that, females in higher condition tended to have fewer nestlings per brood. In addition, neither SMI nor H/L ratio of females were connected to any other aspects of breeding success. Altogether, our results suggest that body condition and age can be relevant to breeding performance of Common Kingfishers but their predictive value is only partial.
Zusammenfassung
Einfluss von Alter und Körperkondition auf den Bruterfolg von Eisvögeln Alcedo atthis
Die Theorie besagt, dass Organismen dazu neigen, ihre Ressourceneinsatzstrategie zu optimieren, und da die Jungenaufzucht kostspielig ist, könnte die Körperkondition ein Indiz für die individuelle Fitness sein, weil Individuen mit besserer Kondition über mehr Ressourcen verfügen, welche sie in die Maximierung ihres Reproduktionserfolges investieren können. Anhand einer dreijährigen Studie (29 Paare, 58 Bruten, 366 Nestlinge) an Eisvögeln Alcedo atthis untersuchten wir, inwiefern das Alter der Elternvögel, der Skalierte Körpermassenindex (engl.: scaled mass index, SMI) und das Verhältnis Heterophilen zu Lymphozyten (H/L-Verhältnis) des Bruterfolg dieser Art beeinflussen. Die Feldarbeit wurde von 2016–2018 am Flusssystem der Donau zwischen Bratislava und Gabčíkovo (Südwest-Slowakei) durchgeführt. Wir stellten fest, dass sich Männchen und Weibchen bezüglich des H/L-Verhältnisses und des SMI unterschieden, wobei die Weibchen eine bessere Gesamtkondition aufwiesen. Gleichzeitig korrelierten weder die beiden Variablen miteinander, noch gab es Unterschiede zwischen Altersgruppen. Weiterführende Analysen zeigten, dass mit einem Männchen mit niedrigerem H/L-Verhältnis verpaarte Weibchen dazu neigten, früher in der Brutsaison mit der Eiablage zu beginnen. Zudem zogen Paare, welche ihre Erstgelege früher in der Brutsaison begonnen hatten, während dieser Saison Nestlinge aus mehr Bruten auf. Auch die Anzahl der Bruten wurde signifikant durch das Alter des Weibchens und den SMI des Männchens beeinflusst, wobei ältere Weibchen und Männchen mit guter Kondition mit einer höheren Wahrscheinlichkeit mehr Bruten hervorbrachten. Im Gegensatz dazu neigten Weibchen mit besserer Kondition dazu, weniger Nestlinge pro Brut zu haben. Darüber hinaus standen weder SMI noch H/L-Verhältnis der Weibchen in irgendeiner Beziehung zu anderen Aspekten des Bruterfolges. Insgesamt legen unsere Ergebnisse nahe, dass Körperkondition und Alter bei Eisvögeln relevant für den Bruterfolg sein können, aber nur teilweise für Vorhersagen geeignet sind.




Similar content being viewed by others
References
Ackerman JT, Hartman CA, Herzog MP (2019) Mercury contamination in resident and migrant songbirds and potential effects on body condition. Environ Pollut 246:797–810
Aloni I, Markman S, Ziv Y (2019) Autumn temperatures at African wintering grounds affect body condition of two passerine species during spring migration. PLoS ONE. https://doi.org/10.1371/journal.pone.0217619
Atkinson CT, van Riper C (1991) Pathogenicity and epizootiology of avian haematozoa: Plasmodium, Leucocytozoon, and Haemoproteus. In: Loye JE, Zuk M (eds) Bird-parasite interactions: Ecology, evolution, and behavior. Oxford University Press, London, pp 19–48
Banbura J, Banbura M, Glądalski M, Kaliński A, Markowski M, Michalski M, Nadolski J, Skwarska J, Zieliński P (2011) Body condition parameters of nestling Great Tits Parus major in relation to experimental food supplementation. Acta Ornithol 46:207–212
Banbura J, Skwarska J, Banbura M, Gladalski M, Holysz M, Kalinski A, Markowski M, Wawrzyniak J, Zielinski P (2013) Spatial and temporal variation in heterophil-to- lymphocyte ratios of nestling passerine birds: comparison of Blue Tits and Great Tits. PLoS ONE. https://doi.org/10.1371/journal.pone.0074226
Barros Á, Álvarez D, Velando A (2013) Climate influences fledgling sex ratio and sex-specific dispersal in a seabird. PLoS ONE. https://doi.org/10.1371/journal.pone.0071358
Bonderud ES, Flood NJ, Van Hamme JD, Boyda CAW, Reudink MW (2016) Female mountain bluebirds (Sialia currucoides) paired to more colourful males produce male-biased broods. Behaviour 153:367–386
Bonneaud C, Mazuc J, Chastel O, Westerdahl H, Sorci G (2004) Terminal investment induced by immune challenge and fitness traits associated with major histocompatibility complex in the House Sparrow. Evolution 58:2823–2830
Booksmythe I, Mautz B, Davis J, Nakagawa S, Jennions MD (2017) Facultative adjustment of the offspring sex ratio and male attractiveness: a systematic review and meta-analysis. Biol Rev 92:108–134
Bouland AJ, White AE, Lonabaugh KP, Varian-Ramos CW, Cristol DA (2012) Female-biased offspring sex ratios in birds at a mercury contaminated river. J Avian Biol 43:244–251
Bradbury RB, Blakey JK (1998) Diet, maternal condition, and offspring sex ratio in the zebra finch, Poephila guttata. Proc R Soc Lond B 265:895–899
Čech P (2010) Length of the breeding season in the Common Kingfisher (Alcedo atthis) in the Czech Republic. Sylvia 46:53–61 (in Czech, English summary)
Čech P (ed) (2007) Common Kingfisher (Alcedo atthhis), its protection and inevstigation. 02/19 ZO Českého svazu ochránců přírody Alcedo, Vlašim (in Czech)
Čech P (2009) Paper on age and sex determination of Kingfisher (Alcedo atthis). In: Čech P (ed) Ledňáček říční (Alcedo atthhis), jeho ochrana a výzkum. Sborník referátů z II. Mezinárodního semináře, Vlašim, pp 118–125 (in Czech, English abstract)
Cepák J, Klvaňa P, Škopek J, Schröpfer L, Jelínek M, Hořák M, Formánek J, Zárybnický J (2008) Atlas migrace ptáků České a Slovenské republiky. Aventinum, Praha (in Czech)
Cepková M, Balážová M, Melišková M, Rubáčová-Turčoková L (2019) No seasonal variation of the sex ratio in the Common Kingfisher Alcedo atthis broods. Acta Ornithol 54:149–155
Clark P, Boardman WSJ, Raidal SH (2009) Atlas of clinical avian hematology. Wiley-Blackwell, New York
Clutton-Brock TH (1984) Reproductive effort and terminal investment in iteroparous animals. Am Nat 123:212–229
Cramp S (ed) (1985) Handbook of Europe the Middle East and North Africa. The birds of the Western Palearctic, vol IV. Oxford University Press, Oxford
Crawford RD (1977) Breeding biology of year-old and older female red-winged and yellow-headed blackbirds. Wilson Bull 89:73–80
Daunt F, Monoghan P, Wanless S, Harris MP, Griffiths R (2001) Sons and daughters: age-differences in parental rearing capacities. Funct Ecol 15:211–216
Davis AK (2005) Effect of handling time and repeated sampling on avian white blood cell counts. J Field Ornithol 76:334–338
De Steven D (1978) The influence of age on the breeding biology of the Tree Swallow Irodoprocne bicolor. Ibis 120:516–523
Demongin L (2016) Identification guide to birds in the hand. Laurent Demongin (Privately published)
Dowling DK, Mulder RA (2006) Combined influence of maternal and paternal quality on sex allocation in red-capped robins. J Evol Biol 19:440–449
Duffield KR, Bowers EK, Sakaluk SK, Sadd BM (2017) A dynamic threshold model for terminal investment. Behav Ecol Sociobiol 71:185
Ellegren H, Gustafsson L, Sheldon BC (1996) Sex ratio adjustment in relation to paternal attractiveness in a wild bird population. Proc Natl Acad Sci 93:11723–11728
Espie RHM, Oliphant LW, James PC, Warkentin IG, Lieske DJ (2000) Age-Dependent Breeding Performance in Merlins (Falco columbarius). Ecology 81:3404–3415
Forslund P, Pärt T (1995) Age and reproduction in birds—hypotheses and tests. Trends Ecol Evol 10:374–378
Frigerio D, Ludwig SC, Hemetsberger J, Kotrschal K, Wascher CAF (2017) Social and environmental factors modulate leucocyte profiles in free-living Greylag geese (Anser anser). PeerJ. https://doi.org/10.7717/peerj.2792
Froy H, Phillips RA, Wood AG, Nussey DH, Lewis S (2013) Age-related variation in reproductive traits in the wandering albatross: evidence for terminal improvement following senescence. Ecol Lett 16:642–649
Gonzáles-Solís J, Becker PH, Jover L, Ruiz X (2004) Individual changes underlie age-specific pattern of laying date and egg-size in female common terns (Sterna hirundo). J Ornithol 145:129–136
Gross WB, Siegel HS (1983) Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Dis 27:972–979
Holmes RT, Marra PP, Sherry TW (1996) Habitat-specific demography of breeding black-throated blue warblers (Dendroica caerulescens): implications for population dynamics. J Anim Ecol 65:183–195
Hunt KL, Fraser JD, Karpanty SM, Catlin DH (2017) Body condition of piping plovers (Charadrius melodus) and prey abundance on flood-created habitat on the Missouri River, USA. Wilson J Ornithol 129:754–764
Kullberg C, Fransson T, Jakobsson S (1996) Impaired predator evasion in fat blackcaps (Sylvia atricapilla). Proc R Soc B Biol Sci 263:1671–1675
Labbé MA, King DI (2020) Songbird use of native and invasive fruit in the Northeastern USA. Wild Soc Bull 44:570–578
Labocha MK, Hayes JP (2012) Morphometric indices of body condition in birds: a review. J Ornithol 153:1–22
Labocha MK, Schutz H, Hayes JP (2014) Which body condition index is best? Oikos 123:111–119
Lee ATK, Barnard P, Fraser M, Lennard C, Smit B, Oschadleus H-D (2020) Body mass and condition of a fynbos bird community: investigating impacts of time, weather and raptor abundance from long-term citizen-science datasets. Ostrich 91:142–157
Legge S, Heinsohn R, Double MC, Griffiths R, Cockburn A (2001) Complex sex allocation in the laughing kookaburra. Behav Ecol 12:524–533
Lima SL (1986) Predation risk and unpredictable feeding conditions: determinants of body mass in birds. Ecology 67:377–385
Lobato E, Moreno J, Merino S, Sanz JJ, Arriero E (2005) Haematological variables are good predictors of recruitment in nestling pied flycatchers (Ficedula hypoleuca). Ecoscience 12:27–34
McCleery RH, Perrins CM, Sheldon BC, Charmantier A (2008) Age-specific reproduction in a long-lived species: the combined effects of senescence and individual quality. Proc R Soc B 275:963–970
McNamara JM, Houston AI, Barta Z, Scheuerlein A, Fromhage L (2009) Deterioration, death and the evolution of reproductive restraint in late life. Proc R Soc B 276:4061–4066
Merkling T, Leclaire S, Danchin E, Lhuillier E, Wagner RH, White J, Hatch SA, Blanchard P (2012) Food availability and offspring sex in a monogamous seabird: insights from an experimental approach. Behav Ecol 23:751–758
Møller AP, Nielsen JT (2014) Parental defense of offspring and life history of a long-lived raptor. Behav Ecol 25:1505–1512
Moreno J, de León A, Fargallo JA, Moreno E (1998) Breeding time, health and immune response in the chinstrap penguin Pygoscelis antarctica. Oecologia 115:312–319
Müller C, Jenni-Eiermann S, Jenni L (2010) Heterophils/Lymphocytes-ratio and circulating corticosterone do not indicate the same stress imposed on Eurasian kestrel nestlings. Funct Ecol 25:566–576
Nilsson J-A, Svensson E (1996) The cost of reproduction: a new link between current reproductive effort and future reproductive success. Proc R Soc Lond B 263:711–714
Nishiumi I (1998) Brood sex ratio is dependent on female mating status in polygynous great red warbler. Behav Ecol Sociobiol 44:9–14
Nol E, Smith JNM (1987) Effects of age and breeding experience on seasonal reproductive success in the Song Sparrow. J Anim Ecol 56:301–313
Norte AC, Araújo PM, Sampaio HL, Sousa JP, Ramos JA (2009) Haematozoa infections in a Great Tit Parus major population in Central Portugal: relationships with breeding effort and health. Ibis 151:677–688
Pärt T (2001) Experimental evidence of environmental effects on age-specific reproductive success: the importance of resource quality. Proc R Soc Lond B 268:2267–2271
Peig J, Green AJ (2009) New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118:1883–1891
Peig J, Green AJ (2010) The paradigm of body condition: a critical reappraisal of current methods based on mass and length. Funct Ecol 24:1323–1332
Peralta-Sánchez JM, Colmenero J, Redondo-Sánchez S, Ontanilla J, Soler M (2019) Females are more determinant than males in reproductive performance in the house sparrow Passer domesticus. J Avian Biol. https://doi.org/10.1111/jav.02240
Perrins CM, Moss D (1974) Survival of young Great Tits in relation to age of female parent. Ibis 116:220–224
Pianka ER, Parker WS (1975) Age-specific reproductive tactics. Am Nat 109:453–464
Pigeault R, Cozzarolo C-S, Glaizot O, Christe P (2019) Effect of age, haemosporidian infection and body condition on pair composition and reproductive success in Great Tits Parus major. Ibis 162:613–626
Poláková R, Schnitzer J, Vinkler M, Bryja J, Munclinger P, Albrecht T (2012) Effect of extra-pair paternity and parental quality on brood sex ratio in the scarlet rosefinch Carpodacus erythrinus. Folia Zool 61:225–232
Price T, Kirkpatrick M, Arnold SJ (1988) Directional selection and the evolution of breeding date in birds. Science 240:798–799
Pugesek BH (1981) Increased reproductive effort with age in the California gull (Larus californicus). Science 212:822–823
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rubáčová L, Melišková M (2020) Extreme breeding effort of Common Kingfisher (Alcedo atthis). Tichodroma. https://doi.org/10.31577/tichodroma.2020.32.1
Rubáčová L, Čech P, Melišková M, Čech M, Procházka P (2021) The effect of age, sex and winter severity on return rates and apparent survival in the Common Kingfisher Alcedo atthis. Ardea 109:15–25
Saalfeld ST, Conway WC, Haukos DA, Johnson WP (2013) Seasonal variation in offspring sex ratio in the snowy plover. West N Am Nat 73:60–71
Sol D, Jovani R, Torres J (2003) Parasite mediated mortality and host immune response explain age-related differences in blood parasitism in birds. Oecologia 135:542–547
Tarwater CE, Arcese P (2017) Age and years to death disparately influence reproductive allocation in a short-lived bird. Ecology 98:2248–2254
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary the sex ratio of offspring. Science 179:90–92
Turčoková L, Melišková M, Balážová M (2016) Nest site location and breeding success of Common kingfisher (Alcedo atthis) in the Danube river system. Folia Oecol 43:74–82
Turčoková L, Melišková M (2017) The first results from the ringing of Common Kingfisher on the Danube river. In: Čech P (ed) Ledňáček říční (Alcedo atthhis), jeho ochrana a výzkum. Sborník referátů z III. Mezinárodního semináře, Vlašim, pp 31–36 (in Czech, English abstract)
Valkiūnas G (2005) Avian malaria parasites and other haemosporidia. CRC Press, Boca Raton
van Oers K, Richardson DS, Sæther SA, Komdeur J (2010) Reduced blood parasite prevalence with age in the Seychelles Warbler: selective mortality or suppression of infection? J Ornithol 151:69–77
Velando A, Drummond H, Torres R (2006) Senescent birds redouble reproductive effort when ill: confirmation of the terminal investment hypothesis. Proc R Soc B 273:1443–1448
Verhulst S, Nilsson JA (2008) The timing of birds’ breeding seasons: a review of experiments that manipulated timing of breeding. Philos Trans R Soc Lond B Biol Sci 363:399–410
Vleck CM, Vertalino N, Vleck D, Bucher TL (2000) Stress, corticosterone, and heterophil to lymphocyte ratios in free-living Adélie penguins. Condor 102:392–400
Wang N, Zhang Z-W (2009) The novel primers for sex identification in the brown eared-pheasant and their application to other species. Mol Ecol Resour 9:186–188
Wappl C, Cimadom A, Filek N, Heyer E, Tebbich S (2020) Under adverse conditions, older small tree finch males (Camarhynchus parvulus) produce more offspring than younger males. Ethology 126:966–975
Wendeln H, Becker PH (1999) Effect of paternal quality and effort on the reproduction of common terns. J Anim Ecol 68:205–214
Westerdahl H, Bensch S, Hansson B, Hasselquist D, von Schantz T (2000) Brood sex ratios, female harem status and resources for nestling provisioning in the great reed warbler (Acrocephalus arundinaceus). Behav Ecol Sociobiol 47:312–318
Westneat DF, Stewart IRK, Halpin Woeste E, Gipson J, Abdulkadir L, Poston JP (2002) Patterns of sex ratio variation in house sparrows. Condor 104:598–609
Wiktander U, Olsson O, Nilsson SG (2001) Age and reproduction in lesser spotted woodpeckers (Dendrocopos minor). Auk 118:624–635
Wilcoxen TE, Boughton RK, Schoech SJ (2010) Selection on innate immunity and body condition in Florida scrub-jays throughout an epidemic. Biol Lett 6:552–554
Woodall P (2001) Family alcedinidae (Kingfishers). In: del Hoyo J, Elliott A, Sargatal J (eds) Handbook of the birds of the world mouse birds to hornbills, vol 6. Lynx Edicions, Barcelona, pp 130–249
Wunderle JM (1991) Age-specific foraging proficiency in birds. Curr Ornithol 8:273–324
Zielińska M, Dubiec A, Zieliński P (2010) Offspring sex ratio skew in the sexually monomorphic house martin Delichon urbicum. J Avian Biol 41:591–596
Acknowledgements
We are grateful to P. Čech for advice with the fieldwork. We would like to thank P. Mikulíček for supervising of laboratory procedures. This research was founded by grants LIFE12 NAT/SK/001137, UK/138/2019, UK/162/2020. Study methods complied with the current laws of the country in which they were performed. Permissions to carry out the study were granted by the relevant national authorities of the Ministry of the Environment of the Slovak Republic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Communicated by I. Moore.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cepková, M., Balážová, M., Melišková, M. et al. Influence of age and body condition on breeding performance in Common Kingfisher Alcedo atthis. J Ornithol 163, 251–261 (2022). https://doi.org/10.1007/s10336-021-01922-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-021-01922-9