Abstract
The Common Crane (Grus grus) population has experienced an unprecedented increase across Europe during the last decades. Although cranes feed mostly on invertebrates, amphibians and berries during the breeding season, they can also eat eggs and young of other birds. Therefore, conservationists have raised concerns about the potential predatory effect of cranes on wetland avifauna, but the effects of crane predation on bird numbers have so far not been investigated. We here test the relationship between the crane and peatland bird population’ abundances in Finland for five common wader and passerine species, and a set of seven less common waders, using line-transect data spanning from 1987 to 2014. We found that the population densities of two small passerines (Meadow Pipit Anthus pratensis and Western Yellow Wagtail Motacilla flava) and one wader species (Wood Sandpiper Tringa glareola) were positively associated with crane numbers, probably related to a protective effect against nest predators. For the two other common species and the set of less common waders, we did not find any significant relationships with crane abundance. None of the species was influenced by the (lagged) effect of crane presence (i.e. years since crane was first observed). Peatland drainage was responsible for most species’ negative densities, indicating the need to protect and restore peatlands to mitigate the loss of peatland bird diversity in Finland. In addition, openness, wetness and area size were important peatland characteristics positively influencing most of the studied bird populations. The development in crane and other mire bird numbers in Europe should be monitored regularly to reveal any possible future predatory effects contributing to the shaping of the peatland bird community.
Zusammenfassung
Bestandsrückgänge bei Moorvögeln stehen nicht im Einklang mit der wachsenden Kranichpopulation
Während der letzten Jahrzehnte hat der Kranich (Grus grus) in Europa ein bisher nicht dagewesenes Populationswachstum erfahren. Obwohl sich Kraniche zur Brutzeit vorwiegend von Wirbellosen, Amphibien und Beeren ernähren, können sie auch die Eier und Küken anderer Vogelarten fressen. Daher haben Naturschützer Bedenken über einen möglichen Prädationseffekt der Kraniche auf die Vogelwelt in Feuchtgebieten geäußert; bislang wurden die Auswirkungen der Prädation durch Kraniche auf die Vogelbestände allerdings noch nicht erforscht. Hier untersuchten wir anhand von Linientransektdaten aus dem Zeitraum von 1987–2014 das Verhältnis zwischen den Populationsbeständen von Kranichen und anderen Moorvögeln – fünf häufige Limikolen- und Singvogelarten sowie einer Gruppe von sieben weniger häufigen Limikolenarten—in Finnland. Es ergab sich, dass die Populationsdichten zweier kleiner Singvogelarten (Wiesenpieper Anthus pratensis und Schafstelze Motacilla flava) sowie einer Limikolenart (Bruchwasserläufer Tringa glareola) in einem positiven Zusammenhang mit der Anzahl der Kraniche standen, was vermutlich auf eine Schutzwirkung vor Nesträubern zurückzuführen ist. Bei den beiden anderen häufigen Arten und der Gruppe weniger häufiger Limikolen ließ sich kein signifikanter Zusammenhang mit den Kranichbeständen feststellen. Keine der Arten wurde durch einen (zeitversetzten) Effekt der Anwesenheit der Kraniche (d. h. der Jahre seit dem ersten Auftreten der Kraniche) im Gebiet beeinflusst. Die Trockenlegung von Mooren zeichnete bei den meisten Arten verantwortlich für negative Dichtewerte, was die Notwendigkeit von Schutz- und Renaturierungsmaßnahmen für Moorgebiete unterstreicht, um dem Diversitätsverlust bei Moorvogelarten in Finnland entgegenzuwirken. Darüber hinaus waren Offenheit, Nässe und die Gebietsgröße der Moore wichtige Merkmale, die auf die meisten der untersuchten Vogelpopulationen eine positive Wirkung hatten. Die Bestandsentwicklungen bei Kranichen und anderen Moorvögeln in Europa sollten regelmäßig dokumentiert werden, um mögliche zukünftige Prädationseffekte aufzudecken, welche Einfluss auf die Zusammensetzung der Vogelgemeinschaften in Moorgebieten haben könnten.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Common Crane (Grus grus, hereafter crane) is a species of conservation importance that has been listed in Annex I of the Birds Directive since 1979 (Nilsson et al. 2019). During the last 40 years, the European population has experienced a significant increase (Vegvari and Tar 2002; Mewes et al. 2010; Harris and Mirande 2013), recovering from a big decline of the breeding population that took place until the 1980s, largely associated with hunting, habitat loss and habitat degradation (European Union 2019; Nilsson et al. 2019). According to the last estimates, the population along the Western European flyway, one of the two major migratory routes used by European cranes (Prange 2005) and the Baltic–Hungarian route (used mostly by north-eastern European cranes; Prange 2005), consists of about 500,000 individuals: 350,000 account for the Western European flyway and 150,000 for the Baltic–Hungarian route (Alonso et al. 2016; Prange 2016). The main reasons responsible for the population increase are the protection and management of many crucial staging, roosting and wintering sites under Natura 2000 (and other funding instruments from the European Union, such as LIFE programmes; e.g. Salvi et al. 1995), landscape-level changes (i.e. creation and restoration of large wetlands) and changed farming practices in the agricultural industry (increased food availability, e.g. due to the intensification of maize cultivation; Nilsson 2002; Mewes et al. 2010; Salvi 2010a, b; European Union 2019).
The crane’s diet during the breeding season (including both breeding and non-breeding birds) mostly consists of invertebrates, amphibians and berries (Nowald 2001; Månsson et al. 2013). Less commonly, cranes can also eat eggs and young of smaller birds, and exceptionally also e.g. adult warblers (Cramp and Simmons 1980). In the Norwegian literature, small birds are mentioned as part of cranes diet (Haftorn 1971), and cases have been documented of cranes stealing and eating the eggs of Common Quails Coturnix coturnix from an artificial nest with camera surveillance (Leistad 2011). Along these lines, concerns have been raised among conservationists about the impact of the increasing crane population on vulnerable birdlife reliant on wetlands due to predation on eggs and chicks (Harvey et al. 1968), and especially in the case of wader species in areas with high crane densities (Nilsson 2016). Despite the increase in crane numbers, evidence of the effects of crane predation on wetland biodiversity is lacking.
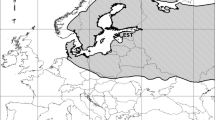
In Finland, cranes are common breeders and passage migrants (Prange 2016). As in other parts of Europe, surveys in Finland have revealed that the national population has increased (Väisänen et al. 1998; Miikkulainen 2001; Leito et al. 2003), and that the breeding-season numbers have increased over six-fold since the early 1980s (Väisänen et al. 2018). The species currently inhabits most parts of Finland (Fig. 1), with an estimate of 37,000–51,000 breeding pairs (Lehikoinen et al. 2019). Breeding cranes were earlier found in rather low densities, generally in large peatlands, but an increasing number of cranes now breed in large reed beds around lakes and sea bays (Karlin 1995; Leito et al. 2005). Extensive drainage of peatlands has been done during the last decades, mostly for improving forestry (Turunen 2008). This has arguably resulted in certain changes in the distribution and habitat use of cranes (Leito et al. 2005). The total drained area is equivalent to 57,000 km2, which is about 60% of the original peatland area (Vasander 1996; Peltola 2004). Because of this practice, combined with large-scale extraction of peat for fuel, nowadays less than 25% of the original peatland area remains in southern and central Finland (Aapala et al. 1996; Auvinen et al. 2007).
Crane distribution in Finnish peatlands based on line-transect data divided into three different periods. The graph depicts only those sites when a crane was first observed: 24 sites (1987–1996), 21 sites (1997–2005) and 261 sites (2006–2014). Note that many new crane observations were detected during the last period, coinciding with the implementation of a systematic sampling
In a recent study carried out by Fraixedas et al. (2017), drainage of mires was found to negatively affect most of the studied species’ densities, since it removes the typical peatland properties beneficial for peatland birds (e.g. openness, wetness, and low tree height). However, the impact of predation on peatland bird populations, such as the effect of cranes, was not examined. Assessing both biotic interactions and environmental factors may provide a more comprehensive understanding of the reasons why species populations decline and/or change their distributions in the landscape, potentially leading to more adequate management solutions (Evans 2004; Andradas et al. 2019; Pass et al. 2019). As a follow-up to the study carried out by Fraixedas et al. (2017), this paper explores how the continuously increasing crane population affects wetland bird abundances based on 28 years of Finnish line-transect data, taking into account the species-specific habitat selection and spatiotemporal trends of five common peatland birds and seven less common peatland wader species. We hypothesize that, if predation by cranes may reduce densities of smaller bird species in peatlands (Berg et al. 1992; Haddad et al. 2000), temporal trends will be more negative in sites after the crane has appeared for the first time, compared to those sites where a crane has not yet appeared. If this is the case, we predict that the species of concern may also avoid areas with many cranes, causing an immediate negative relationship between crane and focal species abundances. Alternatively, bird abundances may be positively linked to crane numbers, if either or both species of concern prefer each other’s company, e.g. if smaller birds enjoy the protection provided by cranes against other predators. Positive or negative effects of this kind can also appear due to similar or different specific habitat selection if the model fails to account for them with the habitat variables included. Furthermore, if no connection between changes in bird numbers is found, we expect the impact of crane predation to be so small that habitat quality is more relevant than predation.
Materials and methods
Study area and habitat data
We used the same classification of peatland habitats as in Fraixedas et al. (2017) and obtained the data from the Finnish line transect bird censuses. This monitoring scheme is coordinated by the Finnish Museum of Natural History with data contributed by volunteers. Censuses were done between late May and early July (but mainly in June), when the vast majority of migratory birds have arrived at their breeding grounds (Koskimies and Väisänen 1991), therefore covering well the focal species of this study. Each route was surveyed only once per breeding season and most often by the same observer across years. Two distance belts are distinguished along the line transect routes: the main belt (25 + 25 m wide) and the supplementary belt, including pairs observed > 25 m from the route and all individuals flying over (Koskimies and Väisänen 1991; Laaksonen and Lehikoinen 2013). Since 1986, habitat data have been recorded from the main belt and structured in twelve habitat categories (see Fraixedas et al. 2015 for additional information). In this study, only information from the habitat categories of forested and open peatland, and their corresponding sub-categories, was considered to extract our habitat-related variables (i.e. openness, wetness, area size, tree height, and two variables related to the drainage of mires). For more information on the selected habitat categories, see Fraixedas et al. (2017). Our dataset included censuses done in 1987–2014, and the observed cranes were presumed to include both breeding and non-breeding birds. There is no clear way to distinguish between them with the line transect census method (i.e. crane numbers were counted during the whole census and we do not necessarily know where the birds breed). Both breeding and non-breeding birds can cause predation, and we assumed that if there were a larger number of cranes observed, they could increase the predation risk in the area.
Study species
We chose the same study species as in Fraixedas et al. (2017), with the exception of Rustic Bunting Emberiza rustica, since it has very different habitat requirements inside the range of peatland habitats compared to the other studied species. Rustic Buntings prefer forest peatlands and edges of natural peatlands (Väisänen and Rauhala 1983; Fraixedas et al. 2017), while all other study species favour interior peatland habitat, which is clearly the habitat of conservation concern. Our five selected species were: Meadow Pipit Anthus pratensis, Common Reed Bunting Emberiza schoeniclus, Western Yellow Wagtail Motacilla flava, Common Snipe Gallinago gallinago and Wood Sandpiper Tringa glareola. We also analysed a set of seven less common waders whose observations were summed: Broad-billed Sandpiper Calidris falcinellus, Ruff Calidris pugnax, Whimbrel Numenius phaeopus, Jack Snipe Lymnocryptes minimus, Red-necked Phalarope Phalaropus lobatus, European Golden Plover Pluvialis apricaria and Spotted Redshank Tringa erythropus. More details on why these species were chosen, and how we determine their general habitat selection, are found in Fraixedas et al. (2017).
Crane observations as explanatory variables
We used the habitat data to extract information on crane abundance “crane.ab” by taking the observations from both the main and supplementary belts for each mire route (i.e. transect). The rationale behind using additional observations from the supplementary belt is that cranes observed outside the main belt may also have a predatory effect within the main belt, as they need large areas to search for food (Cramp and Simmons 1980). Crane observations were extracted in the same way as for the other peatland species. The main difference between crane observations and those calculated for the selected study species was that in the latter case, observations had been previously estimated only from the main belt for each mire route section (e.g. one observation of Wood Sandpiper found in the main belt of 450 m of open wet mires), and not for the whole mire route (see description of mire routes and “site” in Fraixedas et al. 2017). Therefore, the total amount of crane observations found in the main and supplementary belts of a particular mire route was the same for each mire route section. Based on the variable accounting for crane abundance, we created a second variable describing the years elapsed from the first crane observation on a given transect “crane.lag”, being zero in the year when a crane was first observed (i.e. a lagged effect on the trend). In other words, the effect of crane presence defines a partial trend, describing how temporal trends differ in crane sites compared to sites without cranes, and the first presence of crane at a given line-transect represents the turning point of the overall temporal trend.
We omitted cases where routes were counted only once. Given that all variation in crane abundance happened at the transect level, it was important that the random effect of transect was properly estimated, having several observations from all levels modelled as random effects.
Statistical analyses
We first analysed the temporal trend in crane abundance, using crane counts recorded per transect and year. For this, we applied a generalized linear mixed model (GLMM), with a Poisson error distribution and a logarithmic link-function. We placed “year” (continuous variable, centred to zero mean) in the fixed effects part to model the temporal trend, and included “year.f” (factor variable) and “transect” as random effects on the intercept. “Transect” accounts for variation in how good the line transect is for observing cranes on average, and “year.f” accounts for the common between-year variation (inducing spatial synchrony) not explained by the fitted trend. We first included also an observation-level random effect (OLRE) to model the extra-Poisson variation present in the data, but because the estimated standard deviation was zero, we refitted the model excluding the OLRE. To effectively model the trend in crane abundance (observed numbers per counting effort), we added the natural logarithm of the counted transect length as an offset variable in the model. The strength of temporal autocorrelation was tested by extracting the unexplained annual variation (i.e. the random effects of year), and arranging them in a time series to which the autocorrelation function “acf” was applied.
Second, we used the models in Fraixedas et al. (2017), which explain species’ densities using peatland habitat characteristics (openness, wetness, area size, tree height, and two variables related to the drainage of mires) and spatiotemporal trends (latitude, longitude and year, modelling patterns in population densities not explained by habitat), and defined them as the null models. To understand whether crane predation had any effect on species densities, we added to the null models the two crane variables described in the previous section (Table 1). We did this separately for each species and the set of less common waders. All models’ structures followed a separate generalized linear mixed model (GLMM) with a logarithmic link-function and Poisson error distribution. There were differences between the covariates included in each species’ model (Table 1). Similarly, some species’ models included an OLRE accounting for the within-site extra-Poisson variation εis, whereas all models included the among-site random variation as (i.e. variation at the mire-route section level; Table 1). Therefore, the full model (the one including all habitat variables, the spatiotemporal trends and the two random effects) was altered in several cases.
All models were fitted in R software version 3.6.1 (R Core Team 2018) using the package lme4 (Bates et al. 2015).
Results
Crane abundance showed a significant log-linear increase (b = 0.075, SE = 0.010, Z value = 7.33, p value < 0.0001; Fig. 2), which corresponds to an annual increase of 7.8%, or an eightfold increase during the 28-year study period. The estimated random-effect standard deviations of transect and year were 1.333 and 0.126, respectively. There was no relevant autocorrelation in the annual unexplained variation, the estimated correlation with lag 1 being < 0.1.
In general terms, crane abundance had statistically significant positive effects in half of the cases, whereas we did not find any (lagged) effect of crane presence on the trend direction for any of the species. The non-significant effects related to crane occurrence implies that they did not provide additional information beyond the spatiotemporal trends and habitat-related variables included in the models (Table 1). The species with a significant positive immediate effect of crane abundance were Meadow Pipit, Western Yellow Wagtail and Wood Sandpiper (Meadow Pipit: b = 0.101, SE = 0.035, Z value = 2.86, p value = 0.004; Western Yellow Wagtail: b = 0.084, SE = 0.031, Z value = 2.68, p value = 0.007; Wood Sandpiper: b = 0.066, SE = 0.033, Z value = 2.01, p value = 0.044; Table 2).
Compared to the null models (in Fraixedas et al. 2017), we also detected a few differences in the habitat selection and spatiotemporal trends of some species after including the two crane variables. For Meadow Pipit, the positive effect of area size in open peatlands, which was earlier found to be significant, was not detected in this case (Table 2). In addition, this species showed an almost significant latitudinal density shift northwards during the period 1987−2014 (b = 0.058, SE = 0.034, Z value = 1.68, p value = 0.093; Table 2). For Wood Sandpiper, the latitudinal shift northwards was now only a tendency (b = 0.074, SE = 0.038, Z value = 1.93, p value = 0.054; Table 2). For the set of less common waders, the positive effects of both wetness and area size were reinforced and now statistically significant (wetness: b = 0.426, SE = 0.201, Z value = 2.11, p value = 0.035; area size: b = 0.400, SE = 0.167, Z value = 2.39, p value = 0.017; Table 2), while they were previously only tendencies. All other effects related to drainage were maintained (see Fraixedas et al. 2017 to view the results of the aforementioned null models).
Discussion
In this study, we explored possible interspecific interactions based on the idea that both breeding and non-breeding cranes may act as predators on peatland bird species, therefore having an impact on their populations. As an extension of the work carried out by Fraixedas et al. (2017), we tested whether crane abundance and the (lagged) effect of crane presence on the trend direction could explain variation in densities of peatland bird species while also considering habitat variables and spatiotemporal trends. The effect of cranes on wetland bird’s population dynamics is poorly known. Our work aimed to contribute with scientific knowledge about this subject. The three detected effects of cranes were in all cases positive and concerned immediate effects rather than changes in the direction of the temporal trend.
The significant positive effects of crane abundance on Meadow Pipit, Western Yellow Wagtail and Wood Sandpiper seem to reflect habitat selection or nesting place, rather than long-term population changes. These results could be related to a positive ecological interaction between cranes and passerines/waders, with the formation of a protective nesting association (Polak 2014). This kind of associations often involve an aggressive species (e.g. owl or raptor, and in our case cranes) and a less aggressive species, like is the case of a passerine or a wader, which builds its nest close by taking advantage of the defensive behaviour of the protecting species (Polak 2014). This protective effect against nest predators (Tornberg et al. 2016) has been observed for instance between Northern Lapwings Vanellus vanellus and precisely Western Yellow Wagtail and Meadow Pipit in southwest Sweden, an association that the authors described as an anti-predator adaptation (Eriksson and Götmark 1982). The anti-predator adaptation was also pointed out by Tryjanowski (2001), who found that the presence of Northern Raven Corvus corax had a positive impact on the breeding bird community of open farmland in western Poland, including the Western Yellow Wagtail. In Finland and Norway, the breeding Northern Goshawk Accipiter gentilis has a higher abundance of breeding open cup nesters close to its nests than further away, probably because the species poses little risk to small songbirds that benefit from protection against nest predators (Mönkkönen et al. 2007). It is worth highlighting the possibility that these results may simply reflect similar habitat selection in crane, Meadow Pipit, Western Yellow Wagtail and Wood Sandpiper on the focal spatial scale. However, such a habitat effect can only be caused by variation that cannot be explained with the habitat covariates included in the model.
As for peatland habitat-related variables and spatiotemporal trends, Boström and Nilsson (1983) found that most wader densities were higher in larger and wetter open raised bogs, which corresponds with our results of the area having a positive effect on less common wader densities and wetness being an important factor for all our study wader species. Interestingly, the models including the crane variables reinforced in some cases the peatland habitat selection shown by birds (e.g. for the set of less common waders), strengthening the idea that openness, wetness, and area size (the latter particularly relevant in the case of less abundant waders) are common peatland characteristics benefitting species densities (Järvinen and Sammalisto 1976; Väisänen et al. 1998). Conversely, tree height had a negative effect on almost all study species. All other effects related to drainage were maintained as in Fraixedas et al. (2017), and Common Snipe and the set of less common waders were the only species not affected by ditching in forest mires. The new tendency detected for Meadow Pipit showing shifts in species distribution towards northern latitudes, a pattern followed by some of the study species (Western Yellow Wagtail and Wood Sandpiper), could possibly indicate the effects of climate change (Chen et al. 2011) and a higher level of habitat degradation in southern Finland (Virtanen et al. 2003). The density shift signals of climate change on peatland bird species are still relatively weak at present (Fraixedas et al. 2017), although climate change could incur in great degradation in the future (Virkkala et al. 2008). In fact, the coupled effects of climate change and peatland habitat degradation could be arguably responsible for worsening the current population levels of peatland species, as has already been identified in other habitats (e.g. Burns et al. 2016).
Given that the current panorama is being favourable to cranes, the population will likely continue to increase, and knowledge of the impact on wetland biodiversity and the ecological factors determining variation in predation rates may become relevant to species’ conservation. For example, non-breeding cranes congregating in large numbers outside peatland habitats, such as other wetlands or agricultural areas, may locally contribute with predatory effects not covered by this study.
To conclude, cranes seem to have positive effects on a subset of the peatland bird species populations investigated. Our results indicate that cranes may benefit some passerine and wader species, perhaps through protection against nest predators. Rather than pointing out strong or obvious effects of the crane, our results reinforce the need to improve the effectiveness of current management actions and strategies in mitigating loss of peatland bird diversity in Finland. Better conservation plans for peatland bird species are vital, especially taking into account that nest predation may increase with peat harvesting (Haddad et al. 2000; Fraixedas et al. 2017). In this sense, programs such as the Helmi Habitat Program launched by the Finnish Ministry of the Environment, which promotes peatland conservation and restoration among other issues, are key to enhance biodiversity and mitigate climate change (Ministry of the Environment 2019).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Aapala K, Heikkilä R, Lindholm T (1996) Protecting the diversity of Finnish mires. In: Vasander H (ed) Peatlands in Finland. Finnish Peatland Society, Vantaa, pp 45–57
Alonso JC, Alonso JA, Onrubia A, Cruz C, Cangarato R, Rocha P (2016) Assessing four decades of wintering crane counts in Spain, Portugal and Morocco. In: Asociación Amigos de Gallocanta (ed) Proceedings of the VIIIth European Crane Conference. Asociación Amigos de Gallocanta, Gallocanta, pp. 28–37
Andradas MX, Arizaga J, Rodríguez-Pérez J (2019) Species co-occurrence and environmental factors and their effect on the distribution of forest birds in mature forests. Forestry 92:568–576
Auvinen A-P, Hildén M, Toivonen H, Primmer E, Niemelä J, Aapala K, Bäck S, Härmä P, Ikävalko J, Järvenpää E et al (2007) Evaluation of the Finnish National Biodiversity Action Plan 1997–2005. Monographs of the Boreal Environment Research 29, Finnish Environment Institute, Helsinki
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Berg Å, Nilsson SG, Boström U (1992) Predation on artificial wader nests on large and small bogs along a south-north gradient. Ornis Scand 23:13–16
Boström U, Nilsson SG (1993) Latitudinal gradients and local variations in species richness and structure of bird communities on raised peat-Bogs in Sweden. Ornis Scand 14:213–226
Burns F, Eaton MA, Barlow KE, Beckmann BC, Brereton T, Brooks DR, Brown PMJ, Al Fulaij N, Gent T, Henderson I et al (2016) Agricultural management and climatic change are the major drivers of biodiversity change in the UK. PLoS ONE 11(3):e0151595
Chen IC, Hill JK, Ohlemüller R, Roy DB, Thomas CD (2011) Rapid range shifts of species associated with high levels of climate warming. Science 333:1024–1026
Cramp S, Simmons KEL (1980) The birds of the Western Palearctic, Vol. 2: Hawks to Bustards. Oxford University Press, Oxford, London, New York
Eriksson MOG, Götmark F (1982) Habitat selection: do passerines nest in association with Lapwings Vanellus vanellus as defence against predators? Ornis Scand 13(3):189–192
European Union (2019) The birds directive: 40 years conserving our shared natural heritage. Publications Office of the European Union, Luxembourg
Evans KL (2004) The potential for interactions between predation and habitat change to cause population declines of farmland birds. Ibis 146:1–13
Fraixedas S, Lindén A, Lehikoinen A (2015) Population trends of common breeding forest birds in southern Finland are consistent with trends in forest management and climate change. Ornis Fenn 92:187–203
Fraixedas S, Lindén A, Meller K, Lindström Å, Keišs O, Kålås JA, Husby M, Leivits A, Leivits M, Lehikoinen A (2017) Substantial decline of Northern European peatland bird populations: consequences of drainage. Biol Conserv 214:223–232
Haddad S, Desrochers A, Savard J-PL (2000) Artificial nest predation in bogs: does peat harvest increase risk? Ecoscience 7(1):32–37
Haftorn S (1971) Norway birds. Universitetsforlaget, Oslo (In Norwegian)
Harris J, Mirande C (2013) A global overview of cranes: status, threats and conservation priorities. Chin Birds 4(3):189–209
Harvey JM, Lieff BC, MacInnes CD, Prevett JP (1968) Observations on behaviour of sandhill cranes. Wilson Bull 80(4):421–425
Järvinen O, Sammalisto L (1976) Regional trends in the avifauna of Finnish peatland bogs. Ann Zool Fenn 13:31–43
Karlin A (1995) Nesting of cranes in Finland. In: Prange H (ed) Crane research and protection in Europe. Martin-Luther-Universität, Halle-Wittenberg, pp 145–148
Koskimies P, Väisänen RA (1991) Monitoring bird populations: a manual of methods applied in Finland. Zoological Museum,Finnish Museum of Natural History, Helsinki
Laaksonen T, Lehikoinen A (2013) Population trends in boreal birds: continuing declines in agricultural, northern, and long-distance migrant species. Biol Conserv 168:99–107
Lehikoinen A, Below A, Jukarainen A, Laaksonen T, Lehtiniemi T, Mikkola-Roos M, Pessa J, Rajasärkkä A, Rusanen P, Sirkiä P et al (2019) Breeding population sizes of Finnish birds. Linnut-vuosikirja 2018:38–45 (In Finnish with English summary)
Leistad AG (2011) Effect of camera on predation rate of artificial bird nests. Bachelor thesis, Nord-Trøndelag University College (In Norwegian)
Leito A, Truu J, Leivits A, Ojaste I (2003) Changes in distribution and numbers of the breeding population of the Common Crane Grus grus in Estonia. Ornis Fenn 80:159–171
Leito A, Ojaste I, Truu J, Palo A (2005) Nest site selection of the Eurasian Crane Grus grus in Estonia: an analysis of nest record cards. Ornis Fenn 82:44–54
Månsson J, Nilsson L, Hake M (2013) Territory size and habitat selection of breeding Common Cranes (Grus grus) in a boreal landscape. Ornis Fenn 90(2):65–72
Mewes W, Prange H, Nowald G (2010) Current status of the common crane in Germany—breeding, resting and colour banding. In: Nowald G, Weber A, Fanke J, Weinhardt E, Donner N (ed) Proceedings of the VIIth European Crane Conference. Crane Conservation Germany, Groß Mohrdorf, pp 22–29
Miikkulainen A (2001) Population size of breeding cranes Grus grus in Finland. Linnut-vuosikirja 36(3):6–9 (In Finnish with English summary)
Ministry of the Environment (2019) The Helmi Habitat Program enhances biodiversity. Ministry of the Environment of Finland, Helsinki. https://www.ym.fi/helmi. Accessed 19 Dec 2019 (In Finnish)
Mönkkönen M, Husby M, Tornberg R, Helle P, Thomson RL (2007) Predation as a landscape effect: the trading off by prey species between predation risks and protection benefits. J Anim Ecol 76:619–629
Nilsson L (2002) Numbers of mute swans and whooper swans in Sweden, 1967–2000. Waterbirds 25:53–60
Nilsson L (2016) Common cranes in agricultural landscapes—linking space use and foraging patterns to conservation and damage prevention. Dissertation, Swedish University of Agricultural Sciences
Nilsson L, Bunnefeld N, Persson J, Žydelis R, Månsson J (2019) Conservation success or increased crop damage risk? The Natura 2000 network for a thriving migratory and protected bird. Biol Conserv 236:1–7
Nowald G (2001) Behaviour of crane (Grus grus) families in their breeding territories in Northeast Germany: parental care and investment. J Ornithol 142(4):390–403
Pass E, Lodjak J, Mägi M, Lõhmus A (2019) Complex habitat patterns create unpredictable nest predation risk—an artificial nest experiment. Ornis Fenn 96:182–193
Peltola A (2004) Finnish statistical yearbook of forestry 2004. Natural Resources Institute Finland, Helsinki (In Finnish)
Polak M (2014) Protective nesting association between the Barred Warbler Sylvia nisoria and the Red-backed Shrike Lanius collurio: an experiment using artificial and natural nests. Ecol Res 29(5):949–957
Prange H (2005) The status of the Common Crane (Grus grus) in Europe—breeding, resting, migration, wintering, and protection. Proc North Am Crane Workshop 9:9–77
Prange H (2016) The word of cranes: life, environment, protection. Distribution of 15 species. MediaNatur Verlag, Martin-Luther-Universität, Halle-Wittenberg (In German)
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org. Accessed 3 Oct 2019
Salvi A, Petit P, Riols C (1995) Programme for the protection of the crane on its migratory route through France. In: Prange H (ed) Crane research and protection in Europe. Conservation of the common crane in Europe—towards a long-term strategy. International conference. Crane Conservation Germany, Martin-Luther-Universität, Halle-Wittenberg, pp 416–429
Salvi A (2010a) Eurasian Crane (Grus grus) and agriculture in France. In: Harris J (ed) Cranes, agriculture and climate change. International Crane Foundation, Baraboo, Wisconsin, pp 65–70
Salvi A (2010b) Eurasian Crane (Grus grus) and climate change in France. In: Harris J (ed) Cranes, agriculture and climate change. International Crane Foundation, Baraboo, Wisconsin, pp 71–76
Tornberg R, Korpimäki V-M, Rauhala P, Rytkönen S (2016) Peregrine Falcon (Falco peregrinus) may affect local demographic trends of wetland bird prey species. Ornis Fenn 93:172–185
Tryjanowski P (2001) Proximity of raven (Corvus corax) nest modifies breeding bird community in an intensively used farmland. Ann Zool Fenn 38:131–138
Turunen J (2008) Development of Finnish peatland area and carbon storage 1950–2000. Boreal Environ Res 13:319–334
Vasander H (1996) Peatlands in Finland. Finnish Peatland Society, Vantaa
Väisänen RA, Rauhala P (1983) Succession of land bird communities on large areas of peatland drained for forestry. Ann Zool Fenn 20:115–127
Väisänen RA, Lammi E, Koskimies P (1998) Distribution, numbers and population changes of Finnish breeding birds. Otava, Helsinki (In Finnish with English summary)
Väisänen RA, Lehikoinen A, Sirkiä P (2018) Monitoring population changes of land bird species breeding in Finland in 1975–2017. Linnut-vuosikirja 2017:16–31 (In Finnish with English summary)
Vegvari Z, Tar J (2002) Autumn roost site selection by the common crane Grus grus in the Hortobagy National Park, Hungary, between 1995–2000. Ornis Fenn 79:101–110
Virkkala R, Heikkinen RK, Leikola N, Luoto M (2008) Projected large-scale range reductions of northern-boreal land bird species due to climate change. Biol Conserv 141:1343–1353
Virtanen K, Hänninen P, Kallinen R-L, Vartiainen S, Herranen T, Jokisaari R (2003) The peat reserves of Finland in 2000. Report of investigation 156. Geological Survey of Finland, Espoo (In Finnish with English summary)
Acknowledgements
Open access funding provided by University of Helsinki including Helsinki University Central Hospital. We thank all volunteers who contributed to data collection and the personnel at the Finnish Museum of Natural History (Luomus) who is in charge of maintaining the databases. Álvaro Fernández-Llamazares provided help with Fig. 1. The Finnish common bird monitoring has been supported by the Finnish Ministry of the Environment. S. Fraixedas was financially supported by a post-doctoral fellowship from the Helsinki Institute of Sustainability Science (HELSUS), and A. Lehikoinen by the Academy of Finland (grant 275606).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data analysis were performed by Aleksi Lehikoinen, Andreas Lindén and Sara Fraixedas. The first draft of the manuscript was written by Sara Fraixedas and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by O. Krüger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fraixedas, S., Lindén, A., Husby, M. et al. Declining peatland bird numbers are not consistent with the increasing Common Crane population. J Ornithol 161, 691–700 (2020). https://doi.org/10.1007/s10336-020-01777-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-020-01777-6