Abstract
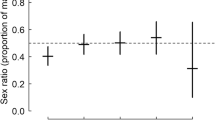
Sex ratio is a fundamental concept in evolutional biology, and theory predicts that parents should invest in sons and daughters according to the fitness returns they expect from them. The fitness returns may depend on the timing of breeding and on parental conditions leading to sex ratios that depend on breeding date and/or parental quality. Here, we investigate the offspring sex ratio in a small shorebird, the Kentish Plover Charadrius alexandrinus, in a large breeding population in Eastern China, and test whether the parents adjust their offspring’s sex in response to hatch date, brood age and their own body condition. Using 1264 chicks from 676 broods that were molecularly sexed, we show that hatchling sex ratio was not significantly different from unity. Hatchling sex ratios were not related to hatch date or to the body condition of parents. In addition, we sexed 138 eggs that were confiscated from illegal egg collectors and found that the mortality of female and male embryos was not significantly different. The latter result is important by suggesting that neither primary sex ratio (i.e., at conception) nor secondary sex ratio (i.e., at hatching) is biased. Taken together, the even offspring sex ratio in Chinese Kentish Plovers is consistent with recent analyses of six plover populations that found even sex ratios at hatching. Future works should investigate whether the even sex ratio persists into adulthood, or it may shift toward more males (or females) due to sex-biased mortalities of juveniles and/or adults.
Zusammenfassung
Das Geschlechterverhältnis des Nachwuchses einer Küstenlimikole ist unabhängig von den Eigenschaften der Eltern und dem Brutzeitbeginn
Das Geschlechterverhältnis ist ein fundamentales Konzept der Evolutionsbiologie, und die Theorie sagt voraus, dass Eltern in Söhne und Töchter proportional zu dem von ihnen erwarteten Fitnessertrag investieren sollten. Der Fitnessertrag hängt möglicherweise vom Brutzeitbeginn und der elterlichen Qualität ab, was dazu führen kann, dass das Geschlechterverhältnis selbst vom Beginn der Brutzeit und den Eigenschaften der Eltern abhängt. Hier untersuchen wir das Geschlechterverhältnis des Nachwuchses einer kleinen Küstenvogelart, dem Seeregenpfeifer Charadrius alexandrinus, in einer großen Brutpopulation in Ostchina und testen, ob die Eltern das Geschlechterverhältnis ihres Nachwuchses dem Schlüpfzeitpunkt, Brutalter und den eigenen körperlichen Bedingungen anpassen. Mittels 1264 Küken aus 676 Bruten, die alle molekular geschlechtsbestimmt waren, zeigen wir, dass das Geschlechterverhältnis nicht signifikant von gleichförmig abwich. Das Geschlechterverhältnis beim Schlupf war weder vom Schlüpfzeitpunkt noch von der Körperkondition der Eltern abhängig. Zusätzlich bestimmten wird das Geschlecht von 138 Eiern, die von illegalen Eisammlern beschlagnahmt wurden, und fanden, dass die Sterblichkeit von weiblichen und männlichen Embryos nicht signifikant verschieden war. Dies zeigt, dass weder das primäre Geschlechterverhältnis (bei der Zeugung) noch das sekundäre Geschlechterverhältnis (beim Schlupf) unausgeglichen sind. Das ausgeglichene Geschlechterverhältnis in den chinesischen Seeregenpfeifern stimmt mit jüngsten Ergebnissen von sechs Regenpfeiferpopulationen überein, die auch ausgeglichene Geschlechterverhältnisse beim Schlupf fanden. Zukünftige Arbeiten sollten untersuchen, ob das ausgeglichene Geschlechterverhältnis bis in das Erwachsenenalter fortbesteht, oder ob es sich zu mehr Männchen (oder Weibchen) verschiebt aufgrund von geschlechtsabhängigen Sterberaten von Jung- und/oder Altvögeln.

Similar content being viewed by others
References
Arroyo B (2002) Sex-biased nestling mortality in the Montagu’s Harrier Circus pygargus. J Avian Biol 33:455–460
Barton K (2015) MuMIn: multi-model inference. http://cran.r-project.org/web/packages/MuMIn/index.html
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bell SC, Owens IP, Lord AM (2014) Quality of breeding territory mediates the influence of paternal quality on sex ratio bias in a free-living bird population. Behav Ecol 25:352–358
Bize P, Roulin A, Tella JL, Richner H (2005) Female-biased mortality in experimentally parasitized Alpine Swift Apus melba nestlings. Funct Ecol 19:405–413
Booksmythe I, Mautz B, Davis J, Nakagawa S, Jennions MD (2017) Facultative adjustment of the offspring sex ratio and male attractiveness: a systematic review and meta-analysis. Biol Rev 92:108–134
Bordier C, Saraux C, Viblanc VA, Gachot-Neveu H, Beaugey M, Le Maho Y, Le Bohec C (2014) Inter-annual variability of fledgling sex ratio in King Penguins. PLoS One 9:e114052
Bowers EK, Thompson CF, Sakaluk SK (2015) Persistent sex-by-environment effects on offspring fitness and sex-ratio adjustment in a wild bird population. J Anim Ecol 84:473–486
Bowers EK, Thompson CF, Sakaluk SK (2017) Maternal natal environment and breeding territory predict the condition and sex ratio of offspring. Evol Biol 44:11–20
Burley N (1981) Sex ratio manipulation and selection for attractiveness. Science 211:721–722
Burnham KP, Anderson DR (2002) Model selection and interference: a practical information-theoretic approach, 2nd edn. Springer, New York
Carmona-Isunza MC, Ancona S, Székely T, Ramallo-González AP, Cruz-López M, Serrano-Meneses MA, Küpper C (2017) Adult sex ratio and operational sex ratio exhibit different temporal dynamics in the wild. Behav Ecol 28:523–532
Cooper NW, Sherry TW, Marra PP (2015) Experimental reduction of winter food decreases body condition and delays migration in a long-distance migratory bird. Ecology 96:1933–1942
Daan S, Dijkstra C, Weissing FJ (1996) An evolutionary explanation for seasonal trends in avian sex ratios. Behav Ecol 7:426–430
Darwin C (1871) The descent of man and selection in relation to sex. Murray, London
Donald PF (2007) Adult sex ratios in wild bird populations. Ibis 149:671–692
Eberhart-Phillips LJ, Küpper C, Miller TE, Cruz-López M, Maher KH, Dos Remedios N, Stoffel MA, Hoffman JI, Krüger O, Székely T (2017) Sex-specific early survival drives adult sex ratio bias in Snowy Plovers and impacts mating system and population growth. Proc Natl Acad Sci USA 117:E5474–E5481
Eberhart-Phillips LJ, Küpper C, Carmona-Isunza MC, Vincze O, Zefania S, Cruz-López M, Kosztolányi A, Miller TE, Barta Z, Cuthill IC, Burke T (2018) Demographic causes of adult sex ratio variation and their consequences for parental cooperation. Nat Commun 9:1651
Ellegren H, Gustafsson L, Sheldon BC (1996) Sex ratio adjustment in relation to parental attractiveness in a wild bird population. Proc Natl Acad Sci USA 93:11723–11728
Fiala KL (1981) Sex ratio constancy in the Red-winged Blackbird. Evolution 35:898–910
Fisher RA (1930) The genetical theory of natural selection: a complete variorum edition. Oxford University Press, Oxford
Fraga RM, Amat JA (1996) Breeding biology of a Kentish Plover (Charadrius alexandrinus) population in an inland saline lake. Ardeola 43:69–85
Fridolfsson AK, Ellegren H (1999) A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 1:116–121
Goławski A, Kasprzykowski Z, Ledwoń M, Mróz E, Morelli F (2016) Brood sex ratio in expansive and non-expansive tern species in east-central Poland. Bird Study 63:31–36
Gomendio M, Clutton-Brock TH, Albon SD, Guinness FE, Simpson MJ (1990) Mammalian sex ratios and variation in costs of rearing sons and daughters. Nature 343:261–263
Hall LK, Cavitt JF (2012) Comparative study of trapping methods for ground-nesting shorebirds. Waterbirds 35:342–346
Hamilton WD (1967) Extraordinary sex ratios. Science 156:477–488
Hardy ICW (2002) Sex ratios: concepts and research methods. Cambridge University Press, Cambridge
Hayman P, Marchant J, Prater T (1986) Shorebirds. An identification guide to the waders of the world. Helm, London
Heinsohn R, Langmore NE, Cockburn A, Kokko H (2011) Adaptive secondary sex ratio adjustments via sex-specific infanticide in a bird. Curr Biol 21:1744–1777
Hewison AJM, Gaillard JM (1999) Successful sons or advantaged daughters? The Trivers-Willard model and sex-biased maternal investment in ungulates. Trends Ecol Evol 14:229–234
Hipkiss T, Hornfeldt B, Eklund U, Berlin S (2002) Year-dependent sex-biased mortality in supplementary-fed Tengmalm’s Owl nestlings. J Anim Ecol 71:693–699
Howe HF (1977) Sex-ratio adjustment in the Common Grackle. Science 198:744–746
Kalmbach E, Benito MM (2007) Sexual size dimorphism and offspring vulnerability in birds. In: Fairbairn DJ, Blanckenhorn WU, Székely T (eds) Sex, size and gender roles: evolutionary studies of sexual size dimorphism. Oxford University Press, Oxford, pp 133–142
Kalmbach E, Furness RW, Griffiths R (2005) Sex-biased environmental sensitivity: natural and experimental evidence from a bird species with larger females. Behav Ecol 16:442–449
Kokko H, Jennions MD (2008) Parental investment, sexual selection and sex ratios. J Evol Biol 21:919–948
Kölliker M, Heeb P, Werner I, Mateman AC, Lessells CM, Richner H (1999) Offspring sex ratio is related to male body size in the Great Tit (Parus major). Behav Ecol 10:68–72
Kosztolányi A, Barta Z, Küpper C, Székely T (2011) Persistence of an extreme male-biased adult sex ratio in a natural population of polyandrous bird. J Evol Biol 24:1842–1846
Küpper C, Augustin J, Kosztolányi A, Burke T, Flguerola J, Székely T (2009) Kentish versus Snowy Plover: phenotypic and genetic analyses of Charadrius alexandrinus reveal divergence of Eurasian and American subspecies. Auk 126:839–852
Lehikoinen A, Öst M, Hollmén T, Kilpi M (2008) Does sex-specific duckling mortality contribute to male bias in adult common eiders? Condor 110:574–578
Lei W (2017) Studies on the waterbirds used saltpans in north of Bohai Bay. Dissertation, Beijing Normal University
Leimar O (1996) Life-history analysis of the Trivers and Willard sex-ratio problem. Behav Ecol 7:316–325
Lessells CM (1984) The mating system of Kentish Plovers Charadrius alexandrinus. Ibis 126:474–483
Lessells CM, Mateman AC, Visser J (1996) Great Tit hatchling sex ratios. J Avian Biol 1:135–142
Liebezeit JR, Smith PA, Lanctot RB, Schekkerman H, Tulp I, Kendall SJ, Tracy DM, Rodrigues RJ, Meltofte H, Robinson JA, Gratto-Trevor C (2007) Assessing the development of shorebird eggs using the flotation method: species-specific and generalized regression models. Condor 109:32–47
Liker A, Freckleton RP, Székely T (2013) The evolution of sex roles in birds is related to adult sex ratio. Nat Commun 4:1587
Liker A, Freckleton RP, Székely T (2014) Divorce and infidelity are associated with skewed adult sex ratios in birds. Curr Biol 24:880–884
Lu X, Zeng X, Du B (2013) Body attributes of both parents jointly affect offspring sex allocation in a socially monogamous, size-monomorphic passerine. Curr Zool 59:271–277
Maddox JD, Weatherhead PJ (2009) Seasonal sex allocation by Common Grackles? Revisiting a foundational study. Ecology 90:3190–3196
McIntosh RR, Kats R, Berg M, Komdeur J, Elgar MA (2003) Breeding ecology and bias in offspring sex ratio in Little Grassbirds (Megalurus gramineus). Aust J Zool 51:505–514
Minias P (2016) Seasonal trends in brood sex ratio reflect changes in early-life physiological condition of chicks in the Whiskered Tern. Ethol Ecol Evol 28:385–393
Myers JH (1978) Sex ratio adjustment under food stress: maximization of quality or numbers of offspring. Am Nat 112:381–388
Orzack SH, Stubblefield JW, Akmaev VR, Colls P, Munné S, Scholl T, Steinsaltz D, Zuckerman JE (2015) The human sex ratio from conception to birth. Proc Natl Acad Sci USA 112:E2102–E2111
Page GW, Quinn PL, Warriner JC (1989) Comparison of the breeding of hand-and wild-reared Snowy Plovers. Conserv Biol 3:198–201
Peig J, Green AJ (2009) New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118:1883–1891
Peig J, Green AJ (2010) The paradigm of body condition: a critical reappraisal of current methods based on mass and length. Funct Ecol 24:1323–1332
Pen I, Weissing FJ, Daan S (1999) Seasonal sex ratio trend in the European kestrel: an evolutionarily stable strategy analysis. Am Nat 153:384–397
Que P (2015) Breeding success and population genetic structure of Kentish Plover Charadrius alexandrinus in China. Dissertation, Beijing Normal University
Que P, Chang Y, Eberhart-Phillips L, Liu Y, Székely T, Zhang Z (2015) Low nest survival of a breeding shorebird in Bohai Bay, China. J Ornithol 156:297–307
Råberg L, Stjernman M, Nilsson JÅ (2005) Sex and environmental sensitivity in Blue Tit nestlings. Oecologia 145:496–503
Ramula S, Öst M, Lindén A, Karell P, Kilpi M (2018) Increased male bias in eider ducks can be explained by sex-specific survival of prime-age breeders. PLoS ONE 13:e0195415
Redfern CPF, Clark JA (2001) Ringers’ manual. British Trust for Ornithology, Thetford
Riordan MM, Lukacs PM, Huyvaert KP, Dreitz VJ (2015) Sex ratios of Mountain Plovers from egg production to fledging. Avian Conserv Ecol 10:3
Robertson BC, Gemmell NJ (2006) PCR-based sexing in conservation biology: wrong answers from an accurate methodology? Conserv Genet 7:267–271
Romano A, Romano M, Caprioli M, Costanzo A, Parolini M, Rubolini D, Saino N (2015) Sex allocation according to multiple sexually dimorphic traits of both parents in the Barn Swallow (Hirundo rustica). J Evol Biol 28:1234–1247
Saalfeld ST, Conway WC, Haukos DA, Johnson WP (2013) Seasonal variation in offspring sex ratio in the Snowy Plover. West N Am Nat 73:60–71
Saunders SP, Cuthbert FJ (2015) Chick mortality leads to male-biased sex ratios in endangered Great Lakes Piping Plovers. J Field Ornithol 86:103–114
Schacht R, Kramer KL, Székely T, Kappeler PM (2017) Adult sex ratios and reproductive decisions: a critical re-examination of sex differences in human and animal societies. Philos Trans R Soc B 372:1729
Sheldon BC, Andersson S, Griffith SC, Örnborg J, Sendecka J (1999) Ultraviolet colour variation influences Blue Tit sex ratios. Nature 402:874–877
Smallwood PD, Smallwood JA (1998) Seasonal shifts in sex ratios of fledgling American Kestrels (Falco sparverius paulus): the early bird hypothesis. Evol Ecol 12:839–853
Stenzel LE, Page GW, Warriner JC, Warriner JS, Neuman KK, George DE, Eyster CR, Bidstrup FC (2011) Male-skewed adult sex ratio, survival, mating opportunity and annual productivity in the Snowy Plover Charadrius alexandrinus. Ibis 153:312–322
Székely T (2014) Sexual conflict between parents: offspring desertion and asymmetrical parental care. In: Rice WR, Gavrilets S (eds) The genetics and biology of sexual conflict. Cold Spring Harbor Laboratory Press, New York, pp 245–263
Székely T, Cuthill IC (1999) Brood desertion in the Kentish Plover: the value of parental care. Behav Ecol 10:191–197
Székely T, Lessells CM (1993) Mate change by Kentish Plovers Charadrius alexandrinus. Ornis Scand 24:317–322
Székely T, Cuthill IC, Kis J (1999) Brood desertion in Kentish Plover: sex differences in remating opportunities. Behav Ecol 10:185–190
Székely T, Cuthill IC, Yezerinac S, Griffiths R, Kis J (2004) Brood sex ratio in the Kentish Plover. Behav Ecol 15:58–62
Székely T, Kosztolányi A, Küpper C (2008) Practical guide for investigating breeding ecology of Kentish Plover Charadrius alexandrinus. University of Bath. http://www.bath.ac.uk/bio-sci/biodiversity-lab/pdfs/KP_Field_Guide_v3.pdf. Accessed 2 Feb 2015
Székely T, Liker A, Freckleton RP, Fichtel C, Kappeler PM (2014a) Sex-biased survival predicts adult sex ratio variation in wild birds. Proc R Soc Lond B 281:20140342
Székely T, Weissing FJ, Komdeur J (2014b) Adult sex ratio variation: implications for breeding system evolution. J Evol Biol 27:1500–1512
Tan LXL, Buchanan KL, Maguire GS, Weston MA (2015) Cover, not caging, influences chronic physiological stress in a ground-nesting bird. J Avian Biol 46:482–488
Trivers RL (1985) Social evolution. Benjamin/Cummings, Menlo Park
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary the sex ratio of offspring. Science 179:90–92
van der Velde M, Haddrath O, Verkuil YI, Baker AJ, Piersma T (2017) New primers for molecular sex identification of waders. Wader Study 124:147–151
Weatherhead PJ (1983) Secondary sex ratio adjustment in Red-winged Blackbirds (Agelaius phoeniceus). Behav Ecol Sociobiol 12:57–61
West S (2009) Sex allocation. Princeton University Press, Princeton
Whittingham LA, Dunn PO (2000) Offspring sex ratios in Tree Swallows: females in better condition produce more sons. Mol Ecol 9:1123–1129
Wiersma P, Kirwan GM, Boesman P (2018) Kentish Plover (Charadrius alexandrinus). In: del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E (ed) Handbook of the birds of the world alive. Lynx, Barcelona. http://www.hbw.com/node/53835. Accessed 22 Jan 2018
Yang H, Chen B, Barter M, Piersma T, Zhou C, Li F, Zhang Z (2011) Impacts of tidal land reclamation in Bohai Bay, China: ongoing losses of critical Yellow Sea waterbird staging and wintering sites. Bird Conserv Int 21:241–259
Acknowledgements
This study was supported by the National Natural Science Foundation of China (nos. 31600297 and 31572288). We especially thank Zhiwei Tian and Jianli Song, who provided much help in the fieldwork. We are grateful to Yajing Chang, Bingrun Zhu, Jin Liu, Jia Zheng, Boshi Liang, Siyuan Huang, Karen Kim, Siyao Zhong, Zhuoxue Chen, Guang Yang, Pei Luo, Christopher Dudley, Carrie Wendt, Rebecca Gouge, Robert Weber, Kai Chen, Xiaoyan Long, Nan Zhang, and Xuecong Zhang for field assistance. We also thank Xunqiang Mo and Jianmin Wang for intercepting the illegal egg collectors and bringing the confiscated eggs to us. We thank Dr. Benjamin Werner for translating the abstract to German. Tamás Székely was a fellow of the Advanced Institute of Berlin at the time of writing the manuscript, and his work was funded by a Royal Society Wolfson Merit Award (WM170050), and by the Hungarian scientific funding agency, NKFIH (ÉLVONAL KKP-126949, K-116310). All experiments described in this study comply with current Chinese laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Krüger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Que, P., Székely, T., Wang, P. et al. Offspring sex ratio is unrelated to parental quality and time of breeding in a multiple-breeding shorebird. J Ornithol 160, 443–452 (2019). https://doi.org/10.1007/s10336-018-1620-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-018-1620-6