Abstract
We studied the differences between spring and winter sites of Hazel Grouse Tetrastes bonasia in a managed, temperate forest in the Beskid Mountains (Western Carpathians, Poland). The study of seasonal requirements of this species in this type of habitat was innovatory. Spring territories must provide birds with appropriate conditions for breeding and winter ones must give good habitat for wintering. The environmental variables of spring and winter sites were collected in three layers in a 100 m radius from the place of recording of the Hazel Grouse: canopy, understory and undergrowth. According to our study, the proportion of deciduous trees was greater in spring territories in comparison to winter territories in all studied layers. Conversely, greater species richness in the undergrowth and understory in spring sites of Hazel Grouse was found in comparison to winter sites. Moreover, a greater proportion of birch, poplar, willow, wild cherry and bird cherry were found in the spring sites of Hazel Grouse in comparison to winter sites. Generalized Linear Model models showed that the occurrence of overgrown clearings, clearfellings with deadwood and higher richness of grass and herbs and their cover in the forest were important habitat factors for Hazel Grouse in both spring and winter sites. Sites of Hazel Grouse were strongly affected by the proportions of beech, sycamore, fir, spruce and larch in the canopy layer in both seasons. Moreover, a greater proportion of tree species producing nuts, drupes or winged seeds was important in both spring and winter sites. Bush cover was important for winter sites of the Hazel Grouse. Food resources, mainly in winter sites are important factors for habitat selection. Summarizing, we found habitat differences between spring territory and winter sites of Hazel Grouse. In both seasons, higher habitat heterogeneity was an important factor for occurrence of this species.
Zusammenfassung
Jahreszeitliche Änderungen in den Habitatansprüchen von Haselhühnern Tetrastes bonasia in bewirtschafteten Bergwäldern (Westkarpaten) Wir untersuchten Unterschiede zwischen den Frühjahr- und Wintervorkommen von Haselhühnern Tetrastes bonasia in einem Wirtschaftswald der gemäßigten Zone in den Beskiden (Westkarpaten, Polen). Eine solche Studie der jahreszeitlichen Bedürfnisse der Art in diesem Lebensraumtyp war etwas völlig Neues. Im Frühjahr müssen die Reviere den Vögeln geeignete Bedingungen für die Brut bieten, im Winter ein entsprechendes Überwinterungshabitat. Auf drei Ebenen bestimmten wir die Umweltvariablen von Frühjahrs- und Winterrevieren in einem Radius vom 100 m um den jeweiligen Beobachtungsort von Haselhühnern: Kronendach, Unterholz und Unterwuchs. Unserer Studie zufolge war auf allen Ebenen der Anteil an Laubbäumen in den Frühjahrsrevieren im Vergleich größer als in den Winterrevieren. Umgekehrt war in den Frühjahrsgebieten der Haselhühner eine höhere Artenvielfalt in Unterholz und Unterwuchs zu finden als in den Wintergebieten. Außerdem gab es in den Frühjahrsgebieten im Vergleich zu den Wintergebieten der Haselhühner einen größeren Anteil an Birke, Pappel, Weide, Vogelkirsche und Traubenkirsche. Generalisierte Lineare Modelle (GLM) zeigten, dass das Vorkommen bewachsener Lichtungen, von Rodungsflächen mit Totholz und eine höhere Vielfalt von Gräsern und Kräutern sowie der Grad der Bodenbedeckung im Wald sowohl bei den Frühjahrs- als auch bei den Wintergebieten wichtige Lebensraumfaktoren für Haselhühner darstellten. Von Haselhühnern genutzte Gebiete wurden zu beiden Jahreszeiten stark vom Anteil an Buche, Sykomore, Tanne, Fichte und Lärche am Kronendach beeinflusst. Außerdem war sowohl für die Frühjahrs- als auch für die Wintergebiete ein größerer Anteil an Nüsse, Steinfrüchte oder Flügelsamen produzierender Baumarten von Bedeutung. Die Bedeckung mit Gebüsch war wichtig für Überwinterungsgebiete der Haselhühner. Nahrungsquellen waren hauptsächlich in den Wintergebieten wichtige Faktoren bei der Habitatwahl. Insgesamt stellten wir Lebensraumunterschiede zwischen Frühjahrsrevieren und Überwinterungsgebieten der Haselhühner fest. Zu beiden Jahreszeiten war eine höhere Lebensraumheterogenität ein wichtiger Faktor für das Vorkommen dieser Vogelart.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hazel Grouse Tetrastes bonasia is generally considered to be a sedentary, forest-specialist bird (Bergmann et al. 1982). It is a medium-sized species, with brown patterned plumage, living mainly on the ground inhabiting coniferous and mixed forests of Eurasia (Bergmann et al. 1996). This grouse occurs in both lowlands and in mountains (Cramp and Simmons 1980). It can live in both natural and extensively managed forests. Hazel Grouse also occupy the early areal stages of forests from small to large scales. It prefers large dense coniferous and mixed complexes, but can also inhabit fragmented, smaller forest complexes, generally with an area exceeding 400 ha (Kajtoch et al. 2012). Such areas include those disturbed naturally or anthropogenically (e.g., resulting from fires, windthrow, snow damage, insect infestation, overgrown clearings and abandoned land) (Bergmann et al. 1996) and areas of rejuvenation embedded in old-growth forests (Swenson et al. 1995; Sachot et al. 2003).
The number and range of Hazel Grouse populations in most European countries has moderately decreased since 1980 (Swenson and Danielson 1991; Storch 2000). This species is listed in the Appendix of the European Birds Directive, mentioned in the Bern Convention and is included in the Carpathian list of endangered species and is threatened in Poland, Czech Republic and Slovakia (Witkowski et al. 2003). The geographical range of Hazel Grouse in the Polish Carpathians is relatively broad and covers all major mountains, while the species is less prevalent in the foothills (Kajtoch et al. 2011; Matysek 2016). A trend for the size of the Polish Carpathian population is difficult to determine, due to the lack of constant monitoring. However, it is known that this population has remained stable in the past with a record of a slight increase in numbers and recolonization of abandoned areas (Bonczar 2009; Kajtoch et al. 2011; Matysek 2016).
Hazel Grouse is a territorial bird with specific habitat and food requirements (Johnsgard 1983; Bergmann et al. 1996). Habitat structure is one of the most important features for habitat selection by birds (Cody 1981). In the spring breeding season, the area of Hazel Grouse territories ranges from approximately 20–35 ha, but can sometimes reach 70 ha (Johnsgard 1983; Swenson 1991a; Bergmann et al. 1996; Montadert and Leonard 2006). Boreal populations of Hazel Grouse tend to migrate on more southern wintering sites, due to a deficiency of food, high predatory in breeding sites and general extreme environmental conditions (Swenson et al. 1995). Contrarily, mountain populations in Europe are sedentary; however, it is uncertain if and how spring and winter territories overlap. In winter sites, Hazel Grouse territories are much smaller and range from approximately 2–16 ha (Pynnönen 1954; Bergmann et al. 1978; Bonczar 2009; Kämpfer-Lauenstein 1995). In small winter patch sites, Hazel Grouse must find food and shelter, which are generally located within the territories of nesting sites or the surrounding border area (Swenson 1991b). Food of the Hazel Grouse consists of seeds, berries, flowers, fruits, shoots, and invertebrates (Cramp and Simmons 1980). The habitat requirements of Hazel Grouse have been investigated in several studies in boreal and temperate forests of Northern and Central Europe (e.g., Kämpfer-Lauenstein 1997; Åberg et al. 2003; Mathys et al. 2006; Müller et al. 2009; Schäublin and Bollman 2011; Ludwig and Klaus 2017). Habitat of Hazel Grouse has been studied in different forest types: mixtures of spruce Picea sp., beech Fagus sp. and fir Abies sp., spruce and pine Pinus sp., mixed beech–spruce, fir–beech, fir–spruce, and pure beech or spruce. However, forest structure and composition in Fennoscandia and the Alps differ strongly from those in the Carpathian Mountains. Generally, Hazel Grouse prefers mature stands with a canopy of tall trees, such as spruce, fir, and larch Larix sp., but with smaller species such as alder Alnus sp. and birch Betula sp. or willow Salix sp. in clearings (Cramp and Simmons 1980). Winter or autumn habitats of Hazel Grouse that were studied in forests in Switzerland, the Czech Republic and South Korea (Sachot et al. 2003; Rhim 2006; Schäublin and Bollman 2011; Ludwig and Klaus 2017). Rhim and Lee (2002) showed that Hazel Grouse used similar areas from spring to autumn but made a shift in their habitat use in winter in forests of South Korea. Similarly, territories of Spruce Grouse Falcipennis canadensis differ in spring and in winter (Allan 1985). Winter is a challenging period for individuals, due to high predation risk, shortage of food, and extreme environmental conditions (Swenson et al. 1995; Yang et al. 2011). Winter habitats of Hazel Grouse were not studied in the Western Carpathians. Differences in the spring and winter habitats of this species within the same population in the Carpathian Mountains have not yet been studied. These territories serve different purposes: spring sites provide proper conditions for breeding, whereas winter sites provide proper conditions for wintering.
Winter can be a very difficult and demanding period for the survival of Hazel Grouse, especially in heavily managed forests. During winter, food and shelter availability can be decreased by a reduction in the heterogeneity of the environment (Johnsgard 1983; Bergmann et al. 1996; Swenson and Olsson 1991; Montadert and Leonard 2003). According to Seibold et al. (2013), forest vegetation structure and reduced heterogeneity of the environment (e.g., no windthrow or bark beetle infestation) could result in greater predation on nests. Habitat loss and deterioration are considered the most important threat to biological diversity, thus the accurate analysis of species–habitat relationships is indispensable for animal conservation. The low density of Hazel Grouse in managed forests, as compared to the density in less intensively managed forests, clearly shows the impact of forest management on the species (Åberg et al. 2003), which are considered the main reasons for reducing the size of Hazel Grouse populations (Storch 2013). As shown in Kajtoch et al. (2012), habitat quality is more important for Hazel Grouse than habitat quantity. Studying the habitat requirements of these birds can lead to better habitat selection and thus aid in their protection (Müller et al. 2009). This could help in the recovery of those Hazel Grouse populations in decline that has occurred in most West European countries, because it is considered as a keystone or umbrella species for heterogeneous forests, including these of a semi-natural character (Pakkala et al. 2014).
The Carpathian Mountain ecosystems in Central Europe are generally regarded as hotspots of biodiversity and priority regions (Bálint et al. 2011). In Poland, these forests provide habitats for a multitude of species. Forest-dwelling specialists are often seriously threatened, especially in regions where landscape transformation and forest fragmentation are well advanced. Unfortunately, heterogeneity of the environment has decreased in most of the Carpathian forests. To aid in the conservation of Hazel Grouse, it is necessary to develop methods appropriate to forest management in the Carpathian Mountains. These forests (mainly conifer forests transformed by inappropriate forest management without taking into consideration habitat forest characteristics) are characteristically different from those in other parts of Europe, where such studies have previously been performed. Forest management methods require knowledge of habitat requirements in both spring and winter.
The aim of this study was to quantify the habitat requirements of Hazel Grouse in temperate forests (mixed forests with dominance of conifers) in the Carpathian Mountains in two seasons. For this purpose, we (1) assessed the distribution of the species in spring and winter, (2) measured habitat variables in three layers of forest in occupied territories, and (3) compared habitat variables in spring and winter sites. This research is innovatory because habitat requirements of Hazel Grouse have not been studied in temperate forest yet. No information is available about seasonal differences of habitat structure in sites occupied by this species in Western Carpathians. Moreover, winter habitats of Hazel Grouse were studied only in a few areas.
Methods
Study area
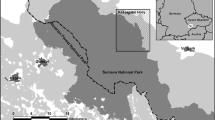
The study was carried out in the eastern part of the Beskid Makowski Mountains (Western Carpathians) (49.48°N, 19.51°E) in southern Poland near Myślenice town ca. 45 km from Krakow (Fig. 1). This area constitutes moderately high hills (altitude: 450–857 m a.s.l.) covered by forests and overgrowing open areas (fields and meadows). After World War II, the abandonment of agricultural land due to changes in management of agriculture, forestry and economic change, resulted in secondary succession of forests, which played a significant role in shaping the environment of the mountains and increasing the cover of the forest (Kozak 2010). Plot area in these forests is very diverse (Kozak 2010). For example, land belonging to the Polish Forestry Services is the largest at a few hectares, whereas private forests have smaller plot areas, ranging from several acres to approximately 1 ha (http://rdlpkrakow.gis-net.pl/). Such diversity in forest size provides a large area of habitat heterogeneity, which is reflected in the large number of grouse in intensively managed forests (Kajtoch et al. 2011).
Different types of forests occurred in the study area: Abieti-Piceetum montanum, Dentario glandulosae-Fagetum westcarp. montane, Luzulo luzuloidis-Fagetum or Galio-Abietenion (Matuszkiewicz 2008). Most forest stands were extensively managed. Forests were mixed with varying proportions of spruce Picea abies, pine Pinus sylvestris, fir Abies alba, beech Fagus sylvatica, birch Betula spp. and other trees that have a high demand for light that covered more than 50% of the study area. Forest patches were located mostly on steep slopes on the tops of hills and in valleys. The forested area consisted of a mosaic of woodland patches. Forests belonged to either the Polish Forestry Services (cn. 30%) or private owners (cn. 70%). State forests were overgrown mainly by beech, fir, and spruce, while private forests were overgrown mainly by spruce and fir, followed by pine and beech. The forests varied in age, and the age of stands was more variable in state-owned forests. The oldest forests were at least 80 years old, contained dying trees and dead wood, and occurred mainly on steep rocky slopes. These types of forests occupied approximately 20% of the total forest area. Forest stands were younger in private forests. The mean age of trees was c. 60 years in state forests and c. 40 years in private ones. The wood resources were c. 290 m3 ha−1 in state forests. There is no such data for private forests, but it is lower by at least 20% in comparison to state forests. Sustainable forests management is carried out and economically valuable species are preferred in state forests. The young trees are planted in the area of clearfelling. Forest management is intensive in private forests. Plantings are not made and the forest renews itself naturally. In many places pastures, meadows, and fields were overgrown and juvenile forest communities consisting of different species (birch, poplar Populus sp., willow Salix sp., hazel Corylus avellana, sycamore Acer pseudoplatanus, and spruce) occurred. Villages and agricultural elements were mostly localised along valleys. Snow cover of variable thickness usually occurred from the end of November until March.
Birds’ places
Places of potential occurrence of the Hazel Grouse were selected based on previous sightings by the authors of grouse in those locations, and analysis of satellite images and topographic maps. The selected places were visited at least twice in both 2009 and 2010 to find Hazel Grouse territories. Similar to Ludwig and Klaus (2017), when assigning locations as territories, we used the term ‘‘Hazel Grouse site” or “site” instead of territory because most Hazel Grouse presence in winter was mostly identified by indirect evidence of presence and not by territorial activities of the birds. To detect Hazel Grouse in spring (April and May) and autumn (October), whistle lures were used as an imitation of Hazel Grouse calls in selected places of potential occurrence. Hazel Grouse presence was tested every 150 m, with a pause of a few minutes to lure the Hazel Grouse with a whistle-pipe, hand-made from a hen bone (Bonczar 2009). After a few minutes of listening, the observer moved to the next point. The census was performed mainly during mornings and evenings because a lower response frequency was found during midday, and only in good weather conditions (Swenson 1991b) (without heavy rain or snow and strong winds). The recorded territories were mapped in the field. Additionally, traces of Hazel Grouse presence, such as excrement, were found. Whistle lures were used in autumn to determine the location of winter sites, which they are much smaller than the territories in autumn, and were most often located in the same area as autumn territories (Swenson 1991b). Luring in autumn was helpful later in the winter to search for sites in winter. Winter sites were selected from autumn territories and confirmed by direct observations of birds or clues, traces or droppings left by the birds.
In 2009 and 2010, we examined 19 known Hazel Grouse spring territories and 16 winter sites for presence of the species and recorded vegetation parameters (habitat variables) at the sites. It is c. 4% of the local population in Beskid Makowski Mountains estimated from 450–500 pairs (Kajtoch et al. 2011). Description of habitat for spring territories was carried out in the sites where birds answered on imitation of its calls in spring of the same year. The description of winter habitat sites in the absence of snow cover was performed in the winter or, in the case of heavy snow cover, in the next spring.
Environmental data
Environmental variables at the local scale in detected spring and winter sites of Hazel Grouse were recorded to explain how habitat features influence site occupancy in two seasons (Table 1). A total of 47 environmental variables were collected for each site (within a 100 m radius) for habitat and three layers of forest (undergrowth, understory and canopy). The proportion (%) of the following taxa were determined: spruce, pine, fir, larch Larix decidua, oak Querqus sp., beech, birch, hornbeam Carpinus betulus, ash Fraxinus excelsior, sycamore, linden Tilia sp., alder Alnus sp., poplar, hazel, willow, elm Ulmus sp., viburnum Viburnum sp., rowan Sorbus aucuparia, hawthorn Crataegus sp., wild cherry Prunus avium, bird cherry Prunus padus, beige Sambucus sp., and juniper Juniperus sp. Other environmental and habitat variables were also determined and analysed: proportion (%) of deciduous tree species, proportion (%) of coniferous species, tree species richness of undergrowth/understory/canopy layer, tree cover (%) of undergrowth/understory/canopy layer, wood cover (%), stand age (proportion of trees <40, 41–80, 81–120, >121 years old), grass and herb richness (<10, 11–20, >21 species), grass and herb cover (0–25, 26–50, >51%), hollows (presence/absence), deadwood (presence/absence), ravines (presence/absence), stream (presence/absence), overgrown clearing, clearfelling, unpaved roads, slope rating, distance to forest edge, property. Age of trees was identified using a map of tree stands. Tree species in undergrowth were determined visually by taking into account tree species in understory and canopy layers.
Statistical analyses
Statistical differences of factors between spring and winter sites of Hazel Grouse were assessed using the Mann–Whitney test. A Chi-squared test was used to compare number of sites where: (1) grass and herbs richness was <10; 10–20, >20 species and (2) grass and herbs cover was 0–25, 26–50, >50%, between spring and winter. To assess the importance of environmental variables in spring and winter sites of Hazel Grouse, univariate models were built using a Generalized Linear Model (GLM) with a binomial error distribution and logit-link function distribution. Principal Component Analysis was used to check collinearity among the environmental variables (Freckleton 2011). Correlated factors were classified to one group to reduce the number of factors for inclusion in the GLM model. Akaike’s information criterion (AIC) was used for model selection (Burnham and Anderson 2004). The resulting models were subsequently ranked in order of increasing QAIC. The model with the lowest QAIC score and highest weight (w) was taken as the most parsimonious, as it explains most of the variance with the fewest parameters. Statistical significance was accepted at p < 0.05. Akaike weights of all models for particular variables were calculated.
In the analysis DEADW + HOLLOWS was joined and determined as DEADWOOD, and RAVINES + STREAMS as VALLEY. Finally, all collected variables were subject to Principal Component Analysis, and the following variables were joined by several separate components for the spring territories and winter sites (Appendix 1 in Supplementary materials).
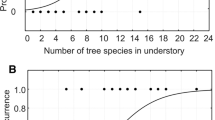
QAIC weights were summed for models containing given variables. The predictor with highest weights (QAIC w) was considered as the most important. Univariate logistic regression modeling was adopted to build curves showing the relationship between the number of tree species and the presence of Hazel Grouse in places. The curves were built for canopy, understory and undergrowth layers. A multimodel inference, made by summing QAIC weights for models containing given variables, was used to assess the real importance of each independent variable (Burnham and Anderson 2004; Freckleton 2011). For the statistical analyses, we used STATISTICA version 12 (StatSoft Inc. 2014).
Results
We found differences between the spring and winter sites of Hazel Grouse in managed, temperate forests of the Beskid Makowski Mountains. The proportion of deciduous trees was greater in spring sites in comparison to winter ones in the canopy layer (Z = 2.748, p < 0.006, N = 35) in understory (Z = 2.798, p < 0.005, N = 35) and in undergrowth (Z = 3.974, p < 0.001, N = 35) (Appendix 2 in Supplementary materials). Inversely, the proportion of coniferous trees was greater in winter sites of the studied species (Appendix 2 in Supplementary materials). Winter sites of this species were characterised by a greater proportion of spruce and fir in the canopy layer (Z = 2.913, p < 0.004, N = 35; Z = 2.359, p < 0.02, N = 35, respectively) and the understory layer (Z = 2.320, p < 0.02, N = 35; Z = 2.590, p < 0.01, N = 35, respectively) (Appendix 2 in Supplementary materials). Greater tree species richness of the undergrowth (Z = 1.954, p < 0.05, N = 35) and understory (Z = 2.235, p < 0.03, N = 35) was found in spring sites of Hazel Grouse compared to winter sites (Appendix 2 in Supplementary materials). Moreover, a greater proportion of birch and poplar were found in the undergrowth (Z = 1.970, p < 0.049, N = 35; Z = 3.041, p < 0.002, N = 35, respectively) and understory (Z = 2.205, p < 0.03, N = 35; Z = 2.081, p < 0.04, N = 35, respectively) in the spring sites of Hazel Grouse in comparison to winter sites (Appendix 2 in Supplementary materials). A greater proportion of willow (Z = 2.697, p < 0.007, N = 35) and wild cherry in undergrowth (Z = 2.136, p < 0.03, N = 35) and bird cherry in the understory (Z = 2.430, p < 0.01, N = 35) of spring sites compared with winter sites was found (Appendix 2 in Supplementary materials). The relative relations between selected factors and Hazel Grouse sites in spring and winter were visualised in Fig. 2.
Comparison of proportion of deciduous trees and tree richness in the forest layers (Mann–Whitney test) and number of sites in three categories of grass and herbs richness and cover (Chi-squared test) in the Hazel Grouse sites in spring and winter. Significant differences were determined: <0.05 as asterisk, <0.01 as “double asterisk”, <0.001 as “triple asterisk”. Nonsignificant differences were determined as “ns”
Model selection
GLM analysis of multivariate models showed that the most parsimonious models included many factors in both spring and winter sites of the Hazel Grouse (Table 2). The model showed that the most important environmental factors for occurrence of Hazel Grouse in spring sites were a complex of the following factors: overgrown clearing, grass and herbs richness and stand age <40 years (∑ QAIC w = 0.96), occurrence of deadwood (∑ QAIC w = 0.96), grass and herbs cover (∑ QAIC w = 0.78), stand age >81 years (∑ QAIC w = 0.55) and clearfelling (∑ QAIC w = 0.39). Similar factors were the most important in winter sites: overgrown clearing with high grass and herb richness (∑ QAIC w = 0.97), occurrence of valley with grass end herb cover (∑ QAIC w = 0.63), deadwood (∑ QAIC w = 0.60) and clearfelling with young trees (age <40 years) (∑ QAIC w = 0.39). The most important factors in the canopy layer in spring sites were the proportion of fir (∑ QAIC w = 0.96), sycamore (∑ QAIC w = 0.88), beech (∑ QAIC w = 0.84), larch (∑ QAIC w = 0.78), and spruce (∑ QAIC w = 0.65). A complex of birch, poplar, willow, wild cherry and alder (∑ QAIC w = 0.54) was the most important in this layer in winter sites. In the understory, a complex of birch, alder, poplar, hazel, willow and rowan (∑ QAIC w = 0.99) was the most important in spring sites. A complex of birch, poplar, hazel, willow and rowan (∑ QAIC w = 0.98) and bush cover (∑ QAIC w = 0.55) and occurrence of alder (∑ QAIC w = 0.53) had the highest importance for winter sites in understory. In the undergrowth, occurrence of spruce (∑ QAIC w = 0.89), fir (∑ QAIC w = 0.85) and a complex of birch, alder, poplar, hazel, willow, wild cherry and rowan (∑ QAIC w = 0.61) were the most important factors in spring sites. Conversely, a cover of tree species (∑ QAIC w = 0.99) and greater occurrence of beech (∑ QAIC w = 0.96) and spruce (∑ QAIC w = 0.57) was important in the undergrowth in winter sites.
The bivariate response surface for the bush cover shows that winter sites of Hazel Grouse are strongly and positively shaped that give greater food resources (Fig. 3a). Probability of Hazel Grouse occurrence did not depend of bush cover in spring. The probability of occurrence of Hazel Grouse in spring territories and winter sites was the highest for areas with high grass and herb cover (Fig. 3b). This suggests that the sheltering function of the forest is less important than food resources in habitat, mainly in winter sites. Grass and herb cover was important in winter, and it is beneficial to some level in spring. Too dense cover decreased probability of Hazel Grouse occurrence probably by too high a risk of predation.
Discussion
The Hazel Grouse has been considered to be a habitat specialist and is present only in forest stands that have been little transformed (Swenson and Danielson 1991; Swenson and Angelstam 1993). The structure of the forest is crucial for the distribution and abundance of many species of birds (Storch 2013; Lycke et al. 2011; Zhang et al. 2013). Models showed that a complex of some factors is important for occurrence of Hazel Grouse in spring and winter. The correct structure of the forest gives a better chance of survival in critical times of the year such as in spring (breeding season) and winter (e.g., less food). Hazel Grouse prefers vertically and horizontally richly structured forest stands. Our results confirmed that habitat differentiation in three layers of forest (canopy, understory and undergrowth) plays an important role in spring territories or winter sites for Hazel Grouse in the Carpathian Mountains. Open areas (overgrown clearing, clearfelling) with dead wood and hollows, and areas rich in grass and herbs are important for Hazel Grouse both in spring and winter territories in managed forests of the Western Carpathians. Kajtoch et al. (2012) showed that among the indices of habitat quality, the most important factors were the presence of clearings and pioneer trees in the Carpathian Foothills. The importance of a shrub layer, especially in earlier succession stages of mixed woodland was also confirmed in east Poland (Wiesner et al. 1977). The occurrence of dead trees and hollows provides shelter from predators or provides sites to build nests and can consequently lead to higher reproductive success (Montadert and Leonard 2004; Seibold et al. 2013). Predation in commercial forests is higher than in natural ones (Seibold et al. 2013). Low habitat heterogeneity and a simplified forest structure increase the possibility of moving and searching for prey by predators. Predation rates decline with increasing density of near-ground vegetation (Seibold et al. 2013).
The occurrence of trees in the understory is an important source of food in both seasons in the Beskid Makowski Mountains. Grass and herb cover is an important factor in the spring territories, because they provide a rich food base. The presence of bilberries was also an important factor for occurrence of Hazel Grouse in the Carpathian Foothills in spring (Kajtoch et al. 2012). Ludwig and Klaus (2017) recently found that the presence of bilberry was beneficial to the Hazel Grouse in the Bohemian Forest (Czech Republic) in the post-breeding period.
A greater proportion of deciduous trees were found in spring territories of Hazel Grouse in our study. In boreal forests (south-central Sweden), sites most often occupied by Hazel Grouse were middle-aged or old stands with a greater proportion of deciduous trees and with a rich field layer (Åberg et al. 2003). We found the importance of high species richness and high proportion of birch, willow, poplar in spring territories in undergrowth and in understory in the studied area. Generally, Hazel Grouse consume flowers and leaves of birch, alder, aspen, willow, and linden, shoots of herbs and small bushes in spring (Glutz von Blotzheim et al. 1973). Similar to the present results, forest edge density, shrub and herb cover, stand structure and development stage were essential habitat variables in Jura Mountains (Switzerland) (Mathys et al. 2006). Declines in the amount of natural deciduous forest in South Korea may be harmful to Hazel Grouse populations, but may also be beneficial by creating denser understory coverage in coniferous plantations (Rhim 2006).
A greater proportion of coniferous trees (mostly pines and firs) and stand density in winter sites provide better conditions to avoid predators and protect against weather conditions (Montadert and Leonard 2004). Deciduous trees lose their leaves and reduce the possibility of hiding from predators in winter. However, dominance of coniferous forest may limit the amount of suitable habitat and, therefore, limit the population density of Hazel Grouse in higher mountain areas (Schäublin and Bollman 2011). Hazel Grouse in the Carpathian Mountains has a very small area of winter site compared to the population residing in Western Europe (Pynnönen 1954; Bergmann et al. 1978; Bonczar 2009; Kämpfer-Lauenstein 1995). In the winter site the occurrence of trees producing seeds, which can be food for Hazel Grouse, decreases the mortality of that species. Birds do not have to search food too far and reduce a risk of being detected by predators. The presence of these two groups of trees may explain the small areas of winter sites in the Western Carpathians. Winter territories were mainly feeding areas containing food resources (Swenson 2006). Food availability is lower in winter than in spring, because some food resources are covered by snow and difficult to find. During winter, catkins, buds, and twigs of birch and alder are consumed by Hazel Grouse (Glutz von Blotzheim et al. 1973). Site occupancy of the Hazel Grouse in the Bohemian Forest in autumn (Czech Republic) was positively influenced by higher proportions of herbs but decreased with a higher proportion of grass cover (Ludwig and Klaus 2017). The winter diet of Hazel Grouse in Central Europe formed the most nutritious food items available in mountain habitats (young buds and catkins of rowan, willow and beech) (Andreev 1988). Swenson (2006) showed that buds and catkins of alder are an important winter food for Hazel Grouse in boreal forests. Sachot et al. (2003) showed that Hazel Grouse preferred feeding sites with a dense understory of rowan, willow, beech and spruce in the upper part (1100–1600 m a.s.l.) of the Jura Mountains (Switzerland). Schäublin and Bollman (2011) showed that Hazel Grouse preferred forests with high proportions of alder and a diverse mosaic of canopy closure and stand structure at the large scale in winter. It preferred stands with high proportion of rowans, forest edges, and dense shrub layer at the small scale. Moreover, the Hazel Grouse uses snow cover and buries itself in it, minimising the lack of places to hide (fallen leaves), reducing predation pressures (Swenson and Olsson 1991).
We found differences between spring and winter habitats of Hazel Grouse in the Beskid Makowski Mountains. Tree species producing nuts, drupes or winged seeds spread out in the private forests, and their share is higher than in state forests where economical valuable tree species were planted in the place of clearfelling. Therefore, Hazel Grouse reached higher density in private forests than in state forests. Some individuals of this species inhabit less favorable areas, but can migrate during the winter to search for a better site as demonstrated by research. Hazel Grouse occurred in flocks in the most suitable habitat during winter in Siberia (Swenson et al. 1995). Åberg et al. (1995) and Montadert and Leonard (2006) showed that individuals usually move from 0.5 to 5 km. Seasonal change in habitats used by Spruce Grouse was found in eastern Maine (USA). During winter, both sexes used conifer stands that were denser than random plots. During spring and summer, females used sites that were less dense than those used by males and had more open canopy and more ground vegetation (Allan 1985).
Key factors of Hazel Grouse distribution are not only environmental factors, but also landscape heterogeneity (Adra et al. 2013). Forest fragmentation has led to a decline in population sizes of forest specialists with limited dispersal abilities. Kajtoch et al. (2012) showed that landscape variables were much more important in explaining the occurrence of Hazel Grouse than variables related to habitat quality in the Carpathian Foothills. We concluded that Hazel Grouse require different sites at different times of the year, and we showed the most important factors in spring and winter territories of the Western Carpathians. In conservation plans for Hazel Grouse and management plans for state and private forestry practices, special attention should be paid to habitats in both spring and winter. In spring territory, Hazel Grouse prefers a greater proportion of deciduous trees in all studied layers, greater species richness in the undergrowth and understory and a greater proportion of trees producing light seeds. In the winter, conifers provide food and protection from predators and adverse weather conditions. Habitat heterogeneity seems to be very important in both seasons. To maintain the Hazel Grouse population in Carpathian forests, the properly management plan should be prepared based on the natural succession, forest renewals, and leaving or introduction of the pioneering species. Moreover, leaving windfallen trees and trees of different ages is important. In addition, the maintenance of clearings within forests, moors, heaths and continuity of forest to maintain migratory routes could be good practice in protecting of Hazel Grouse. Results could be important for management plans and for protection of this species being protected under the Birds Directive.
References
Åberg J, Jansson G, Swenson JE, Angelstam P (1995) The effect of matrix on the occurrence of hazel grouse (Bonasa bonasia) in isolated habitat fragments. Oecologia 103:265–269
Åberg J, Swenson JE, Angelstam P (2003) The habitat requirements of hazel grouse (Bonasa bonasia) in managed boreal forest andapplicability of forest stand descriptions as a tool to identify suitable patches. For Ecol Manag 175:437–444
Adra W, Delcros P, Luque S (2013) Landscape structure indicators as a key feature in habitat selection: an operational approach to conservation planning. J Biodivers Endanger Species 1(2):107. doi:10.4172/2332-2543.1000107
Allan TA (1985) Seasonal changes in habitat use by Maine spruce grouse. Can J Zool 63:2738–2742. doi:10.1139/z85-408
Andreev AV (1988) Ecological energetics of Palearctic Tetraonidae in relation to chemical composition and digestibility of their winter diet. Can J Zool 66:1382–1388
Bálint M, Ujvárosi L, Theissinger K, Lehrian S, Mészáros N, Pauls FU (2011) The Carpathians as a major diversity hotspot in Europe. In: Habel JC, Zachos FE (eds) Biodiversity hotspots. Springer-Verlag, Berlin, pp 189–205
Bergmann HH, Klaus S, Muller F, Wiesner J (1978) Das Haselhuhn, II edn. A. Ziemsen Verlag, Wittenberg-Lutherstadt (in German)
Bergmann HH, Klaus S, Müller F, Wiesner J (1982) Das Haselhuhn Bonasa bonasia Wittenberg. The Hazel Grouse. Die NeueBrehm-Bücherei, Lutherstadt (in German)
Bergmann HH, Klaus S, Müller F, Scherzinger W, Swenson JE, Wiesner J (1996) Die Haselhühner, Bonasa bonasia und B. sewerzowi. Die Neue Brehm-Bücherei, Westrap Wissenschaften, Magdeburg (in German)
Bonczar Z (2009) Hazel Grouse Bonasa bonasia. In: Chylarecki P, Sikora A, Cenian Z (eds) Monitoring of breeding birds. Methodological guide concerning species protected by Birds Directive. Biblioteka Monitoringu Środowiska, Warszawa, pp 287–291 (in Polish)
Burnham KP, Anderson DR (2004) Multimodel inference. Understanding AIC and BIC in model selection. Sociol Method Res 33:261–304
Cody ML (1981) Habitat selection in birds: the roles of vegetation structure, competitors, and productivity. Bioscience 31:107–113
Cramp S, Simmons KEL (1980) The birds of the Western Palearctic. Handbook of the Birds of Europe, the Middle East and North Africa, vol 2. Oxford University Press, Oxford
Freckleton RP (2011) Dealing with collinearity in behavioural and ecological data: model averaging and the problems of measurement error. Behav Ecol Sociobiol 65:91–101
Glutz von Blotzheim UM, Bauer KM, Bezzel E (1973) Handbuch der Vogel Mitteleuropeas, vol 5. Academische Verlagsgesellchaft, Frankfurt am Main (in German)
Johnsgard P (1983) The grouse of the world. University of Nebraska Press, Lincoln
Kajtoch Ł, Matysek M, Skucha P (2011) Forest grouses Tetraoninae of Beskid Wyspowy and Beskid Makowski Mountains and adjacent foothills. Chrońmy Przyr Ojcz 67(1):27–38 (in Polish with English summary)
Kajtoch Ł, Żmihorski M, Bonczar Z (2012) Hazel Grouse occurrence in fragmented forests: habitat quantity and configuration is more important than quality. Eur J For Res 131:1783–1795
Kämpfer-Lauenstein A (1995) Mehr Wildnis für das Haselhuhn! Nationalpark 86:6–9 (in German)
Kämpfer-Lauenstein A (1997) Habitat selection of hazel grouse Bonasa bonasia and natural dynamics in different central European woodland associations. Wildl Biol 3:289
Kozak J (2010) Reforesting landscapes. Reforesting landscapes link. Pattern Process Landsc Ser 10:253–273
Ludwig T, Klaus S (2017) Habitat selection in the post-breeding period by Hazel Grouse Tetrastes bonasia in the Bohemian Forest. J Ornithol 158:101–112. doi:10.1007/s10336-016-1365-z
Lycke A, Imbeau L, Drapeau P (2011) Effects of commercial thinning on site occupancy and habitat use by spruce grouse in boreal Quebec. Can J For Res 41:501–508. doi:10.1139/X10-226
Mathys L, Zimmermann NE, Zbinden N, Suter W (2006) Identifying habitat suitability for hazel grouse Bonasa bonasia at the landscape scale. Wildl Biol 12:357–366
Matuszkiewicz JM (2008) Potential natural vegetation of Poland. IGiPZ PAN, Warszawa (in Polish)
Matysek M (2016) Hazel Grouse (Tetrastes bonasia). In: Pępkowska-Król A, Bobrek R, Wilk T (eds) The birds of the Polish Carpathians—status, threats, conservation. OTOP, Marki, pp 112–120 (in Polish with English summary)
Montadert M, Leonard P (2003) Survival in an expanding Hazel Grouse Bonasa bonasia population in the southeastern French Alps. Wildl Biol 9:357–364
Montadert M, Leonard P (2004) First results of a hazel grouse population study in the south-eastern French Alps. Newslett WPA/BirdLife/Species Surviv Comm Grouse Spec Group 28:15–20
Montadert M, Leonard P (2006) Post-juvenile dispersal of Hazel Grouse Bonasa bonasia in an expanding population of the southeastern French Alps. Ibis 148:1–13
Müller D, Schröder B, Müller J (2009) Modelling habitat selection of the cryptic Hazel Grouse Bonasa bonasia in a montane forest. J Ornithol 150(4):717–732
Pakkala T, Lindén L, Tiainen J, Tomppo E, Kouki J (2014) Indicators of forest biodiversity: which bird species predict high breeding bird assemblage diversity in boreal forests at multiple spatial scales? Ann Zool Fenn 51:457–476
Pynnönen A (1954) Beiträge zur Kenntnis der Lebenweise des Haselhuhns Tetrastes bonasia L. Pap Game Res 12:1–90
Rhim S-J (2006) Home range and habitat selection of hazel grouse Bonasa bonasia in a temperate forest of South Korea. For Ecol Manag 226(1–3):22–25
Rhim S-J, Lee W-S (2002) Characteristics of seasonal movement of hazel grouse (Bonasa bonasia) in a temperate forest. J For Res 13(2):131–134
Sachot S, Perrin N, Neet C (2003) Winter habitat selection by two sympatric forest grouse in western Switzerland: implications for conservation. Biol Conserv 112:373–382
Schäublin S, Bollman K (2011) Winter habitat selection and conservation of Hazel Grouse (Bonasa bonasia) in mountain forests. J Ornithol 152:179–192
Seibold S, Hempel A, Piehl S, Bässler C, Brandl R, Rösner S, Müller J (2013) Forest vegetetion structure has mor influence on predation risk of artificial ground nests than human activities. Basic Appl Ecol 14(8):687–693
StatSoft Inc (2014) STATISTICA (data analysis software system), version 12. http://www.statsoft.pl. Accessed 7 Jan 2016
Storch I (2000) Conservation status and threats to grouse worldwide: an overview. Wildlife Biol 6:195–204
Storch I (2013) Human disturbance of grouse—why and when? Wildlife Biol 19:390–403
Swenson JE (1991a) Social organization of Hazel Grouse and ecological factors influencing it. PhD thesis. University of Alberta; Edmonton
Swenson JE (1991b) Evaluation of the density index for territorial male from Hazel Grouse Bonasa bonasia in spring and autumn. Ornis Fenn 68:57–65
Swenson JE (2006) The importance of alder to Hazel Grouse in Fennoscandian boreal forest: evidence from four levels of scale. Ecography 16(1):37–46
Swenson JE, Angelstam P (1993) Habitat separation by sympatric forest grouse in Fennoscandia in relation to boreal forest succession. Can J Zool 71:1303–1310. doi:10.1139/z93-180
Swenson JE, Danielson J (1991) Status and conservation of Hazel Grouse in Europe. Ornis Scand 22:297–298
Swenson JE, Olsson B (1991) Hazel Grouse night roost site preferences when snow-roosting is not possible in winter. Ornis Scand 22:284–286
Swenson JE, Andreev AV, Drovetskii SV (1995) Factors shaping winter social organization in hazel grouse Bonasa bonasia: a comparative study in the eastern and western Palearctic. J Avian Biol 26:4–12
Wiesner J, Bergmann H-H, Klaus S, Müller F (1977) Siedlungsdichte und Habitatstruktur des Haselhuhns (Bonasa bonasia) im Waldgebietvon Bialowieza (Polen). J Ornithol 118:1–20
Witkowski ZJ, Król W, Solarz W (2003) Carpathian List of Endangered Species. WWF and Institute of Nature Conservation, Polish Academy of Sciences, Vienna-Krakow
Yang C, Fang Y, Sun YH (2011) Winter space use and social behaviors of Chinese grouse (Bonasa sewerzowi) at Lianhuashan mountains, Gansu, China. J Ornithol 152:297–305
Zhang J, Kissling WD, He F (2013) Local forest structure, climate and human disturbance determine regional distribution of boreal bird species richness in Alberta, Canada. J Biogeogr 40:1131–1142. doi:10.1111/jbi.12063
Acknowledgements
We are grateful for valuable comments of two anonymous reviewers, which allowed the improvement of our paper. The study was partly supported by the Institute of Nature Conservation, Polish Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Gottschalk.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Matysek, M., Gwiazda, R. & Bonczar, Z. Seasonal changes of the Hazel Grouse Tetrastes bonasia habitat requirements in managed mountain forests (Western Carpathians). J Ornithol 159, 115–127 (2018). https://doi.org/10.1007/s10336-017-1484-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-017-1484-1