Abstract
Objective
Low-energy shockwave (SW) therapy attenuates damage in the stenotic kidney (STK) caused by atherosclerotic renal artery stenosis (ARAS). We hypothesized that magnetic resonance elastography (MRE) would detect attenuation of fibrosis following SW in unilateral ARAS kidneys.
Materials and methods
Domestic pigs were randomized to control, unilateral ARAS, and ARAS treated with 6 sessions of SW over 3 consecutive weeks (n = 7 each) starting after 3 weeks of ARAS or sham. Four weeks after SW treatment, renal fibrosis was evaluated with MRE in vivo or trichrome staining ex vivo. Blood pressure, single-kidney renal-blood-flow (RBF) and glomerular-filtration-rate (GFR) were assessed.
Results
MRE detected increased stiffness in the STK medulla (15.3 ± 2.1 vs. 10.1 ± 0.8 kPa, p < 0.05) that moderately correlated with severity of fibrosis (R2 = 0.501, p < 0.01), but did not identify mild STK cortical or contralateral kidney fibrosis. Trichrome staining showed that medullary fibrosis was increased in ARAS and alleviated by SW (10.4 ± 1.8% vs. 2.9 ± 0.2%, p < 0.01). SW slightly decreased blood pressure and normalized STK RBF and GFR in ARAS. In the contralateral kidney, SW reversed the increase in RBF and GFR.
Conclusion
MRE might be a tool for noninvasive monitoring of medullary fibrosis in response to treatment in kidney disease.


Similar content being viewed by others
Abbreviations
- SW:
-
Shockwave
- MRI:
-
Magnetic resonance imaging
- MRE:
-
Magnetic resonance elastography
- DWI:
-
Diffusion-weighted imaging
- EPI:
-
Echo-planar imaging
- MEG:
-
Motion encoding gradients
- ROI:
-
Region of interest
- MDCT:
-
Multi-detector computed tomography
- ARAS:
-
Atherosclerotic renal artery stenosis
- STK:
-
Stenotic kidney
- CLK:
-
Contralateral kidney
- RBF:
-
Renal blood flow
- GFR:
-
Glomerular filtration rate
- MAP:
-
Mean arterial pressure
References
Plouin PF, Bax L (2010) Diagnosis and treatment of renal artery stenosis. Nat Rev Nephrol 6:151–159
Low G, Owen NE, Joubert I et al (2015) Reliability of magnetic resonance elastography using multislice two-dimensional spin-echo echo-planar imaging (SE-EPI) and three-dimensional inversion reconstruction for assessing renal stiffness. J Magn Reson Imaging 42:844–850
Hueper K, Khalifa AA, Brasen JH et al (2016) Diffusion-Weighted imaging and diffusion tensor imaging detect delayed graft function and correlate with allograft fibrosis in patients early after kidney transplantation. J Magn Reson Imaging 44:112–121
Jiang K, Ferguson CM, Woollard JR et al (2017) Magnetization transfer magnetic resonance imaging noninvasively detects renal fibrosis in swine atherosclerotic renal artery stenosis at 3.0 T. Invest Radiol 52:686–692
Kline TL, Irazabal MV, Ebrahimi B et al (2016) Utilizing magnetization transfer imaging to investigate tissue remodeling in a murine model of autosomal dominant polycystic kidney disease. Magn Reson Med 75:1466–1473
Ebrahimi B, Rihal N, Woollard JR et al (2014) Assessment of renal artery stenosis using intravoxel incoherent motion diffusion-weighted magnetic resonance imaging analysis. Invest Radiol 49:640–646
Petitclerc L, Sebastiani G, Gilbert G et al (2017) Liver fibrosis: review of current imaging and MRI quantification techniques. J Magn Reson Imaging 45:1276–1295
Greenleaf JF, Fatemi M, Insana M (2003) Selected methods for imaging elastic properties of biological tissues. Annu Rev Biomed Eng 5:57–78
Yin M, Chen J, Glaser KJ et al (2009) Abdominal magnetic resonance elastography. Top Magn Reson Imaging 20:79–87
Rouviere O, Souchon R, Pagnoux G et al (2011) Magnetic resonance elastography of the kidneys: feasibility and reproducibility in young healthy adults. J Magn Reson Imaging 34:880–886
Lee CU, Glockner JF, Glaser KJ et al (2012) MR elastography in renal transplant patients and correlation with renal allograft biopsy: a feasibility study. Acad Radiol 19:834–841
Korsmo MJ, Ebrahimi B, Eirin A et al (2013) Magnetic resonance elastography noninvasively detects in vivo renal medullary fibrosis secondary to swine renal artery stenosis. Invest Radiol 48:61–68
Cooper CJ, Murphy TP, Cutlip DE et al (2014) Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 370:13–22
Textor SC (2011) Issues in renovascular disease and ischemic nephropathy: beyond ASTRAL. Curr Opin Nephrol Hypertens 20:139–145
Meier P, Rossert J, Plouin PF et al (2007) Atherosclerotic renovascular disease: beyond the renal artery stenosis. Nephrol Dial Transplant 22:1002–1006
Eirin A, Zhu XY, Urbieta-Caceres VH et al (2011) Persistent kidney dysfunction in swine renal artery stenosis correlates with outer cortical microvascular remodeling. Am J Physiol Renal Physiol 300:F1394–F1401
Fukumoto Y, Ito A, Uwatoku T et al (2006) Extracorporeal cardiac shock wave therapy ameliorates myocardial ischemia in patients with severe coronary artery disease. Coron Artery Dis 17:63–70
Wang CJ, Huang KE, Sun YC et al (2011) VEGF modulates angiogenesis and osteogenesis in shockwave-promoted fracture healing in rabbits. J Surg Res 171:114–119
Aicher A, Heeschen C, Sasaki K et al (2006) Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: a new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation 114:2823–2830
Zhang X, Krier JD, Amador Carrascal C et al (2016) Low-Energy shockwave therapy improves ischemic kidney microcirculation. J Am Soc Nephrol 27:3715–3724
Stulak JM, Lerman A, Porcel MR et al (2001) Renal vascular function in hypercholesterolemia is preserved by chronic antioxidant supplementation. J Am Soc Nephrol 12:1882–1891
Chade AR, Rodriguez-Porcel M, Grande JP et al (2002) Distinct renal injury in early atherosclerosis and renovascular disease. Circulation 106:1165–1171
Muftuler LT (2013) Quantifying morphology and physiology of the human body using MRI. CRC Press, Boca Raton
Sinkus R, Tanter M, Xydeas T et al (2005) Viscoelastic shear properties of in vivo breast lesions measured by MR elastography. Magn Reson Imaging 23:159–165
Warner L, Yin M, Glaser KJ et al (2011) Noninvasive In vivo assessment of renal tissue elasticity during graded renal ischemia using MR elastography. Invest Radiol 46:509–514
Kotecha M, Magin RL, Mao JJ (2017) Magnetic resonance imaging in tissue engineering. Wiley, Hoboken
Kline TL, Edwards ME, Garg I et al (2017) Quantitative MRI of kidneys in renal disease. Abdom Radiol (NY). https://doi.org/10.1007/s00261-017-1236-y
Ebrahimi B, Gloviczki M, Woollard JR et al (2012) Compartmental analysis of renal BOLD MRI data: introduction and validation. Invest Radiol 47:175–182
Daghini E, Primak AN, Chade AR et al (2007) Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: comparison with electron-beam CT. Radiology 243:405–412
Lerman LO, Textor SC, Grande JP (2009) Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis 52:196–203
Brodsky SV, Yamamoto T, Tada T et al (2002) Endothelial dysfunction in ischemic acute renal failure: rescue by transplanted endothelial cells. Am J Physiol Renal Physiol 282:F1140–F1149
Johns EJ (2009) Inflammation: the underlying foe in renovascular hypertension? J Hypertens 27:1964–1965
Vaidya VS, Ford GM, Waikar SS et al (2009) A rapid urine test for early detection of kidney injury. Kidney Int 76:108–114
Eddy AA (1998) Interstitial fibrosis in hypercholesterolemic rats: role of oxidation, matrix synthesis, and proteolytic cascades. Kidney Int 53:1182–1189
Zhang H, Sun SC (2015) NF-kappaB in inflammation and renal diseases. Cell Biosci 5:63
Chade AR, Bentley MD, Zhu X et al (2004) Antioxidant intervention prevents renal neovascularization in hypercholesterolemic pigs. J Am Soc Nephrol 15:1816–1825
Eirin A, Ebrahimi B, Zhang X et al (2014) Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc Res 103:461–472
Warner GM, Cheng J, Knudsen BE et al (2012) Genetic deficiency of Smad3 protects the kidneys from atrophy and interstitial fibrosis in 2K1C hypertension. Am J Physiol Renal Physiol 302:F1455–F1464
Gennisson JL, Grenier N, Combe C et al (2012) Supersonic shear wave elastography of in vivo pig kidney: influence of blood pressure, urinary pressure and tissue anisotropy. Ultrasound Med Biol 38:1559–1567
Manotham K, Tanaka T, Matsumoto M et al (2004) Evidence of tubular hypoxia in the early phase in the remnant kidney model. J Am Soc Nephrol 15:1277–1288
Yin M, Talwalkar JA, Glaser KJ et al (2007) Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol 5(1207–1213):e1202
Huwart L, Peeters F, Sinkus R et al (2006) Liver fibrosis: non-invasive assessment with MR elastography. NMR Biomed 19:173–179
Acknowledgements
This study was partly supported by NIH grants numbers HL123160, DK73608, DK104273, HL121561, C06-RR018898. We are grateful to Medispec® LTD, Gaithersburg, MD, for generously allowing the use of the SW machine. The machine sponsor was not involved in data collection or analysis.
Author information
Authors and Affiliations
Contributions
ZX Protocol, data collection, data analysis; ZX Data collection; FC Data collection/data analysis; JK Data analysis; BT Data analysis; LA Protocol; LLO Protocol/project development
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interests.
Ethical standards
All applicable international, national, and/or institutional guidelines for care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. This article does not contain any studies with human participants performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10334_2017_671_MOESM1_ESM.tiff
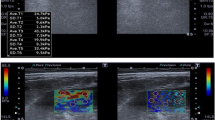
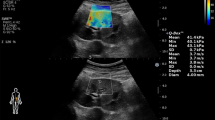
Top: Representative images and quantification of trichrome staining from contralateral medulla. SW ameliorated the increased medullary fibrosis in ARAS CLK. Bottom: Representative MRE images (yellow/red color indicates greater stiffness) from contralateral cortex and medulla (TIFF 2309 kb)
Rights and permissions
About this article
Cite this article
Zhang, X., Zhu, X., Ferguson, C.M. et al. Magnetic resonance elastography can monitor changes in medullary stiffness in response to treatment in the swine ischemic kidney. Magn Reson Mater Phy 31, 375–382 (2018). https://doi.org/10.1007/s10334-017-0671-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-017-0671-7