Abstract
Objective
To demonstrate that high resolution 1H semi-LASER MRSI acquired at 7 T permits discrimination of metabolic patterns of different thalamic nuclei.
Materials and methods
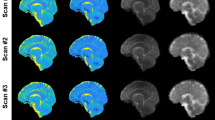
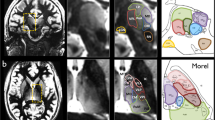
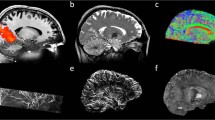
Thirteen right-handed healthy volunteers were explored at 7 T using a high-resolution 2D-semi-LASER 1H-MRSI sequence to determine the relative levels of N-Acetyl Aspartate (NAA), choline (Cho) and creatine-phosphocreatine (Cr) in eight VOIs (volume <0.3 ml) centered on four different thalamic nuclei located on the Oxford thalamic connectivity atlas. Post-processing was done using the CSIAPO software. Chemical shift displacement of metabolites was evaluated on a phantom and correction factors were applied to in vivo data.
Results
The global assessment (ANOVA p < 0.05) of the neurochemical profiles (NAA, Cho and Cr levels) with thalamic nuclei and hemispheres as factors showed a significant global effect (F = 11.98, p < 0.0001), with significant effect of nucleus type (p < 0.0001) and hemisphere (p < 0.0001). Post hoc analyses showed differences in neurochemical profiles between the left and the right hemisphere (p < 0.05), and differences in neurochemical profiles between nuclei within each hemisphere (p < 0.05).
Conclusion
For the first time, using high resolution 2D-PRESS semi-LASER 1H-MRSI acquired at 7 T, we demonstrated that the neurochemical profiles were different between thalamic nuclei, and that these profiles were dependent on the brain hemisphere.






Similar content being viewed by others
References
Morel A, Magnin M, Jeanmonod D (1997) Multiarchitectonic and stereotactic atlas of the human thalamus. J Comput Neurol 387:588–630
Minagar A, Barnett MH, Benedict RH, Pelletier D, Pirko I, Sahraian MA, Frohman E, Zivadinov R (2013) The thalamus and multiple sclerosis Modern views on pathologic, imaging, and clinical aspects. Neurology 80:210–219
Ryan NS, Keihaninejad S, Shakespeare TJ, Lehmann M, Crutch SJ, Malone IB, Thornton JS, Mancini L, Hyare H, Yousry T, Ridgway GR, Zhang H, Modat M, Alexander DC, Rossor MN, Ourselin S, Fox NC (2013) Magnetic resonance imaging evidence for presymptomatic change in thalamus and caudate in familial Alzheimer’s disease. Brain 136:1399–1414
Gan J-L, Cheng Z-X, Duan H-F, Yang J-M, Zhu X-Q, Gao C-Y (2014) Atypical antipsychotic drug treatment for 6 months restores N-acetylaspartate in left prefrontal cortex and left thalamus of first-episode patients with early onset schizophrenia: a magnetic resonance spectroscopy study. Psychiatry Res Neuroimaging 223:23–27
Cifelli A, Arridge M, Jezzard P, Esiri MM, Palace J, Matthews PM (2002) Thalamic neurodegeneration in multiple sclerosis. Ann Neurol 52:650–653
Houtchens MK, Benedict RHB, Killiany R, Sharma J, Jaisani Z, Singh B, Weinstock-Guttman B, Guttmann CRG, Bakshi R (2007) Thalamic atrophy and cognition in multiple sclerosis. Neurology 69:1213–1223
Audoin B, Ranjeva J-P, Au Duong MV, Ibarrola D, Malikova I, Confort-Gouny S, Soulier E, Viout P, Ali-Chérif A, Pelletier J, Cozzone PJ (2004) Voxel-based analysis of MTR images: a method to locate gray matter abnormalities in patients at the earliest stage of multiple sclerosis. J Magn Reson Imaging JMRI 20:765–771
Guye M, Régis J, Tamura M, Wendling F, McGonigal A, Chauvel P, Bartolomei F (2006) The role of corticothalamic coupling in human temporal lobe epilepsy. Brain J Neurol 129:1917–1928
Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM (2003) Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6:750–757
Tourdias T, Saranathan M, Levesque IR, Su J, Rutt BK (2014) Visualization of intra-thalamic nuclei with optimized white-matter-nulled MPRAGE at 7 T. NeuroImage 84:534–545
Cross NE, Lagopoulos J, Duffy SL, Cockayne NL, Hickie IB, Lewis SJG, Naismith SL (2013) Sleep quality in healthy older people: relationship with 1H magnetic resonance spectroscopy markers of glial and neuronal integrity. Behav Neurosci 127:803–810
Yang Z-Y, Yue Q, Xing H-Y, Tan Q-Y, Sun H-Q, Gong Q-Y, Tan Z-J, Quan H (2015) A quantitative analysis of (1)H-MR spectroscopy at 3.0 T of three brain regions from childhood to middle age. Br J Radiol 88:1052
Fojtiková D, Brázdil M, Horký J, Mikl M, Kuba R, Krupa P, Rektor I (2006) Magnetic resonance spectroscopy of the thalamus in patients with typical absence epilepsy. Seizure 15:533–540
Geurts JJG, Reuling IEW, Vrenken H, Uitdehaag BMJ, Polman CH, Castelijns JA, Barkhof F, Pouwels PJW (2006) MR spectroscopic evidence for thalamic and hippocampal, but not cortical, damage in multiple sclerosis. Magn Reson Med 55:478–483
Van Au Duong M, Audoin B, Le Fur Y, Confort-Gouny S, Malikova I, Soulier E, Viout P, Ali-Cherif A, Pelletier J, Cozzone PJ, Ranjeva J-P (2007) Relationships between gray matter metabolic abnormalities and white matter inflammation in patients at the very early stage of MS: a MRSI study. J Neurol 254:914–923
Bertholdo D, Watcharakorn A, Castillo M (2013) Brain proton magnetic resonance spectroscopy: introduction and overview. Neuroimaging Clin N Am 23:359–380
Sen F, Ulubay H, Ozeksi P, Sargon MF, Tascioglu AB (2005) Morphometric measurements of the thalamus and interthalamic adhesion by MR imaging. Neuroanatomy 4:10–12
Bogner W, Gruber S, Trattnig S, Chmelik M (2012) High-resolution mapping of human brain metabolites by free induction decay 1H MRSI at 7 T. NMR Biomed 25:873–882
Považan M, Hangel G, Strasser B, Gruber S, Chmelik M, Trattnig S, Bogner W (2015) Mapping of brain macromolecules and their use for spectral processing of 1H-MRSI data with an ultra-short acquisition delay at 7 T. NeuroImage 121:126–135
Kim D-H, Adalsteinsson E, Glover GH, Spielman DM (2002) Regularized higher-order in vivo shimming. Magn Reson Med 48:715–722
Scheenen TWJ, Klomp DWJ, Wijnen JP, Heerschap A (2008) Short echo time1H-MRSI of the human brain at 3 T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Magn Reson Med 59:1–6
Scheenen TWJ, Heerschap A, Klomp DWJ (2008) Towards 1H-MRSI of the human brain at 7T with slice-selective adiabatic refocusing pulses. Magn Reson Mater Phy Biol Med 21:95–101
Goelman G, Liu S, Fleysher R, Fleysher L, Grossman RI, Gonen O (2007) Chemical-shift artifact reduction in hadamard-encoded MR spectroscopic imaging at high (3 T and 7 T) magnetic fields. Magn Reson Med 58:167–173
Marjańska M, Auerbach EJ, Valabrègue R, Van de Moortele P-F, Adriany G, Garwood M (2012) Localized 1H NMR spectroscopy in different regions of human brain in vivo at 7 T: T 2 relaxation times and concentrations of cerebral metabolites. NMR Biomed 25:332–339
Edden RAE, Schär M, Hillis AE, Barker PB (2006) Optimized detection of lactate at high fields using inner volume saturation. Magn Reson Med 56:912–917
Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM (2012) FSL. NeuroImage 62:782–790
Le Fur Y, Nicoli F, Guye M, Confort-Gouny S, Cozzone PJ, Kober F (2009) Grid-free interactive and automated data processing for MR chemical shift imaging data. Magn Reson Mater Phy Biol Med 23:23–30
Vanhamme L, van den Boogaart A, Van Huffel S (1997) Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129:35–43
de Beer R, van den Boogaart A, van Ormondt D, Pijnappel WW, den Hollander JA, Marien AJ, Luyten PR (1992) Application of time-domain fitting in the quantification of in vivo 1H spectroscopic imaging data sets. NMR Biomed 5:171–178
Pan JW, Lo K-M, Hetherington HP (2012) Role of very high order and degree B 0 shimming for spectroscopic imaging of the human brain at 7 tesla. Magn Reson Med 68:1007–1017
Boer VO, Siero JCW, Hoogduin H, van Gorp JS, Luijten PR, Klomp DWJ (2011) High-field MRS of the human brain at short TE and TR. NMR Biomed 24:1081–1088
Witte ME, Mahad DJ, Lassmann H, van Horssen J (2014) Mitochondrial dysfunction contributes to neurodegeneration in multiple sclerosis. Trends Mol Med 20:179–187
Rae CD (2014) A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem Res 39:1–36
Rovo Z, Ulbert I, Acsady L (2012) Drivers of the primate thalamus. J Neurosci 32:17894–17908
Bracken BK, Jensen JE, Prescot AP, Cohen BM, Renshaw PF, Öngür D (2011) Brain metabolite concentrations across cortical regions in healthy adults. Brain Res 1369:89–94
Steriade M, Deschenes M (1984) The thalamus as a neuronal oscillator. Brain Res 320:1–63
Goldschmidt RC, Hough LB, Glick SD, Padawer J (1984) Mast cells in rat thalamus: nuclear localization, sex difference and left–right asymmetry. Brain Res 323:209–217
Kovács P, Hernádi I, Wilhelm M (2006) Mast cells modulate maintained neuronal activity in the thalamus in vivo. J Neuroimmunol 171:1–7
Ojemann GA (1977) Asymmetric function of the thalamus in man. Ann N Y Acad Sci 299:380–396
Eidelberg D, Galaburda AM (1982) Symmetry and asymmetry in the human posterior thalamus. I. Cytoarchitectonic analysis in normal persons. Arch Neurol 39:325–332
Guillery RW (1995) Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review. J Anat 187:583
Haber SN, Calzavara R (2009) The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull 78:69–74
Kipp M, Wagenknecht N, Beyer C, Samer S, Wuerfel J, Nikoubashman O (2015) Thalamus pathology in multiple sclerosis: from biology to clinical application. Cell Mol Life Sci 72:1127–1147
Wylezinska M, Cifelli A, Jezzard P, Palace J, Alecci M, Matthews PM (2003) Thalamic neurodegeneration in relapsing-remitting multiple sclerosis. Neurology 60:1949–1954
Power BD, Looi JC (2015) The thalamus as a putative biomarker in neurodegenerative disorders. Aust N Z J Psychiatry 49:502–518
Karlsen AS, Korbo S, Uylings HBM, Pakkenberg B (2014) A stereological study of the mediodorsal thalamic nucleus in Down syndrome. Neuroscience 279:253–259
Byne W, Fernandes J, Haroutunian V, Huacon D, Kidkardnee S, Kim J, Tatusov A, Thakur U, Yiannoulos G (2007) Reduction of right medial pulvinar volume and neuron number in schizophrenia. Schizophr Res 90:71–75
Acknowledgments
The authors thank Dr. Malgorzata Marjanska from the CMRR (MI, USA) for the distribution of the semi-LASER sequence. We would like to thank Ben Ridley for useful suggestions on the manuscript. The first author is the recipient of a Ph.D. Grant (CIFRE) supported by Siemens France and the French ‘Association Nationale Recherche et Technologie’ (ANRT). This project is supported by the French IA Equipex 7T-AMI (2011) program ANR-11-EQPX-0001 and the A*MIDEX 7T-AMISTART (2013) through the ANR-11-IDEX-0001-02 program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10334_2016_556_MOESM2_ESM.tiff
Corrected Cho/Cr ratios within homologous thalamic nuclei. * represents significative left/right neurochemical differences for each nucleus [Post-hoc Wilcoxon test (p < 0.05 corrected for multiple comparisons)] 2 (TIFF 258 kb)
Rights and permissions
About this article
Cite this article
Donadieu, M., Le Fur, Y., Confort-Gouny, S. et al. Evidencing different neurochemical profiles between thalamic nuclei using high resolution 2D-PRESS semi-LASER 1H-MRSI at 7 T. Magn Reson Mater Phy 29, 491–501 (2016). https://doi.org/10.1007/s10334-016-0556-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-016-0556-1