Abstract
Object
1H NMR-based metabolic profiling has been used to investigate areas of the heart after an acute myocardial infarction.
Methods
Tissue was obtained from control, at-risk (areas that survive within the infarct zone) and necrotic myocardium after 48 min of left anterior descending coronary artery occlusion and 2 h of reperfusion in a swine model. HR-MAS (high resolution magic angle spectroscopy) spectra from intact tissue and tissue extract spectra were obtained for each region and statistical models were built for each type of spectra allowing differentiation between control, at-risk and necrotic heart.
Results
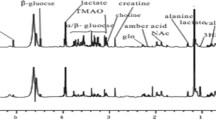
At-risk and, especially, necrotic areas have a reduced concentration of NMR visible metabolites as compared to control tissue, total creatine (phosphorilated and unphosphorilated) being the single most important metabolite in the different discriminant models. Creatine concentration decreased from 18.28 ± 0.84 μmols/g fresh weight in controls to 12.58 ± 2.89 (P < 0.05) and 9.96 ± 2.21 (P < 0.01) in at-risk and necrotic areas, respectively. Taurine and myo-inositol were also involved in the discriminant models. HR-MAS spectra also showed an increase in lipid signals at 0.9 and 1.28 ppm as markers of necrotic tissue. These results support the view that the analysis of in vivo 1H MRS may have value in differentiating normal, at-risk and infarcted myocardium.
Similar content being viewed by others
Abbreviations
- LAD:
-
Left anterior descending coronary artery
- PCs:
-
Principal component(s)
- PCA:
-
Principal component analysis
- PLS-DA:
-
Partial least squares discriminant analysis
- VIP:
-
Variable influence on projection
- HR-MAS:
-
High resolution magic angle spinning
- BSA:
-
Bovine serum albumin
References
Horn M (2006) Cardiac magnetic resonance spectroscopy: a window for studying physiology. Methods Mol Med 124: 225–248
Nakae I, Mitsunami K, Omura T, Yabe T, Tsutamoto T, Matsuo S, Takahashi M, Morikawa S, Inubushi T, Nakamura Y, Kinoshita M, Horie M (2003) Proton magnetic resonance spectroscopy can detect creatine depletion associated with the progression of heart failure in cardiomyopathy. J Am Coll Cardiol 42: 1587–1593
Reingold JS, McGavock JM, Kaka S, Tillery T, Victor RG, Szczepaniak LS (2005) Determination of triglyceride in the human myocardium by magnetic resonance spectroscopy: reproducibility and sensitivity of the method. Am J Physiol Endocrinol Metab 289: E935–E939
Bottomley PA, Weiss RG (2001) Noninvasive localized MR quantification of creatine kinase metabolites in normal and infarcted canine myocardium. Radiology 219: 411–418
Garcia-Dorado D, Oliveras J, Gili J, Sanz E, Perez-Villa F, Barrabes J, Carreras MJ, Solares J, Soler-Soler J (1993) Analysis of myocardial oedema by magnetic resonance imaging early after coronary artery occlusion with or without reperfusion. Cardiovasc Res 27: 1462–1469
Aletras AH, Tilak GS, Natanzon A, Hsu LY, Gonzalez FM, Hoyt RF Jr, Arai AE (2006) Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation 113: 1865–1870
Griffin JL (2003) Metabonomics: NMR spectroscopy and pattern recognition analysis of body fluids and tissues for characterisation of xenobiotic toxicity and disease diagnosis. Curr Opp Chem Biol 7: 1–7
Jones GL, Sang E, Goddard C, Mortishire-Smith RJ, Sweatman BC, Haselden JN, Davies K, Grace AA, Clarke K, Griffin JL (2005) A functional analysis of mouse models of cardiac disease through metabolic profiling. J Biol Chem 280: 7530–75399
Garcia-Dorado D, Gonzalez MA, Barrabes JA, Ruiz-Meana M, Solares J, Lidon RM, Blanco J, Puigfel Y, Piper HM, Soler-Soler J (1997) Prevention of ischemic rigor contracture during coronary occlusion by inhibition of Na(+)-H+ exchange. Cardiovasc Res 35: 80–89
Griffin JL, Sang E, Evens T, Davies K, Clarke K (2002) Metabolic profiles of dystrophin and utrophyin expression in mouse models of Duchenne muscular dystrophy. FEBS Lett 530: 109–116
Backstrom T, Goiny M, Lockowandt U, Liska J, Franco-Cereceda A (2003) Cardiac outflow of amino acids and purines during myocardial ischemia and reperfusion. J Appl Physiol 94: 1122–1128
Straeter-Knowlen IM, Evanochko WT, den Hollander JA, Wolkowicz PE, Balschi JA, Caulfield JB, Ku DD, Pohost GM (1996) 1H NMR spectroscopic imaging of myocardial triglycerides in excised dog hearts subjected to 24 h of coronary occlusion. Circulation 93: 1464–1470
Barba I, Cabanas ME, Arús C (1999) The relationship between nuclear magnetic resonance-visible lipids, lipid droplets, and cell proliferation in cultured C6 cells. Cancer Res 59: 1861–1868
Quintero M, Cabanas ME, Arús C (2007) A possible cellular explanation for the NMR-visible mobile lipid (ML) changes in cultured C6 glioma cells with growth. Biochim Biophys Acta 1771: 31–44
Nakae I, Mitsunami K, Matsuo S, Inubushi T, Morikawa S, Tsutamoto T, Koh T, Horie M (2005) Myocardial creatine concentration in various nonischemic heart diseases assessed by 1H magnetic resonance spectroscopy. Circ J 69: 711–716
Neubauer S (2007) The failing heart. An engine out of fuel. N Engl J Med 256: 1140–1151
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by Red de Enfermedades Cardiovasculares (RECAVA) CICYT SAF2005-01758 and EU Grant FP6-513595.
Rights and permissions
About this article
Cite this article
Barba, I., Jaimez-Auguets, E., Rodriguez-Sinovas, A. et al. 1H NMR-based metabolomic identification of at-risk areas after myocardial infarction in swine. Magn Reson Mater Phy 20, 265–271 (2007). https://doi.org/10.1007/s10334-007-0097-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-007-0097-8