Abstract
Objective
The aim of this study was to construct THY1 eukaryotic expression plasmid and study its effects on ovarian cancer SKOV3 cells.
Methods
The gene fragment coding for THY1 was obtained from human normal ovarian tissue using RT-PCR, and inserted into the eukaryotic expression plasmid pcDNA3.1 (+) to construct the recombinant plasmid pcDNA3.1(+)-THY1, which was transfected into SKOV3 cells. The experimental cells were classified into three groups: SKOV3-THY1, SKOV3-Null and SKOV3. The expression of gene was measured using RT-PCR and Western blotting. The percentage of apoptotic cells and cell cycle analysis and cell proliferation were assessed by flow cytometry and MTT assay. Both SKOV3-THY1 and SKOV3-null cells were inoculated subcutaneously into nude mice to determine in vivo tumorigenicity.
Results
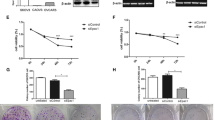
The gene fragment of THY1 was correctly inserted into the eukaryotic expression plasmid pcDNA3.1 (+) and verified by PCR, restriction endonucleases digestion and DNA sequencing and the plasmid of pcDNA3.1(+)-THY1 (THY1 gene overexpression) has been stably transfected into SKOV3 cells. The analysis of flow cytometry indicated that the pcDNA3.1(+)-THY1 transfected cells in G1 phase were significantly elevated, but in S phase were decreased. The growth of transfected cells was suppressed, and more apoptosis cells were identified in pcDNA3.1(+)-THY1 transfectants compared with vector vehicle transfectants. The tumor suppressing activity of THY1 in SKOV3 cells was associated with inhibition of SKOV3 cellular proliferation, in vivo tumorigenesis in nude mice.
Conclusion
THY1 transfection can inhibit the growth of SKOV-3 cells in vitro and in vivo. THY1 gene may play an important role in generation and development of ovarian cancers.
Similar content being viewed by others
References
Seki T, Spurr N, Obata F, et al. The human THY1 gene: structure and chromosomal location. Proc Natl Acad Sci USA, 1985, 82: 6657–6661.
Davis M, Hitchcock A, Foulkes WD, et al. Refinement of two chromosome 11q regions of loss of heterozygosity in ovarian cancer. Cancer Res, 1996, 56: 741–744.
Gabra H, Watson JE, Taylor KJ, et al. Definition and refinement of a region of loss of heterozygosity at 11q23.3–q24.3 in epithelial ovarian cancer associated with poor prognosis. Cancer Res, 1996, 56: 950–954.
Crawford JM, Barton RW. Thy-1 glycoprotein: structure, distribution, and ontogeny. Lab Invest, 1986, 54: 122–135.
Hueber AO, Raposo G, Pierres M, et al. Thy-1 triggers mouse thymocyte apoptosis through a bcl-2-resistant mechanism. J Exp Med, 1994, 179: 785–796.
Abeysinghe HR, Cao Q, Xu J, et al. THY1 expression is associated with tumor suppression of human ovarian cancer. Cancer Genet Cytogenet, 2003: 143: 125–132.
Cao Q, Abeysinghe H, Chow O, et al. Suppression of tumorigenicity in human ovarian carcinoma cell line SKOV-3 by microcell-mediated transfer of chromosome 11. Cancer Genet Cytogenet, 2001, 129: 131–137.
Lee WS, Jain MK, Arkonac BM, et al. THY1, a novel marker for angiogenesis upregulated by inflammatory cytokines. Circ Res, 1998, 82: 845–851.
Beissert S, He HT, Hueber AO, et al. Impaired cutaneous immune responses in THY1-deficient mice. J Immunol, 1998, 161: 5296–5302.
Barker TH, Grenett HE, MacEwen MW, et al. Thy-1 regulates fibroblast focal adhesions, cytoskeletal organization and migration through modulation of p190 RhoGAP and Rho GTPase activity Exp Cell Res, 2004, 295: 488–496.
Diaz-Romero J, Gaillard JP, Grogan SP, et al. Immunophenotypic analysis of human articular chondrocytes: changes in surface markers associated with cell expansion in monolayer culture. J Cell Physiol, 2005, 202: 731–742.
Cavallaro U and Christofori G. Cell adhesion in tumor invasion and metastasis: loss of the glue is not enough. Biochim Biophys Acta, 2001, 1552: 39–45.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by a grant from the Medical Research Foundation of Guangdong Province (No. A2008099).
Rights and permissions
About this article
Cite this article
Zeng, L., Peng, Z. & Luo, X. Growth inhibitory effects of THY1 gene on epithelial ovarian cancer SKOV3 cells. Chin. -Ger. J. Clin. Oncol. 8, 476–480 (2009). https://doi.org/10.1007/s10330-009-0090-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10330-009-0090-y