Abstract
Objective
The aim of the study was to observe the transfection efficacy of hepatitis B virus envelope (HBVE) and evaluate its ability as a gene transfer vector for liver cancer cells.
Methods
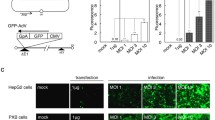
To obtain HBVE, the supernatant fluid of HepG 2.2.15 cells was mixed with a PEG8000 solution for concentration and was inactivated by β-propiolactone. The acquired HBVE was used to pack pIRES2-EGFP to test its package ability. Then, we examined its quantity and quality with ELISA, PCR, SDS-PAGE and electron microscopy. The pIRES2-EGFP was packed with HBVE and obtained the product HBVE-GFP. The pIRES2-EGFP was packed with liposome and obtained the product liposome-GFP. HBVE-GFP and liposome-GFP were used to transfer HepG 2 cells to study the transfection efficiency. HBVE-GFP was used to transfer HepG 2, A549, HeLa and FB cells to study the targeting ability. The green fluorescent protein (GFP) expression was observed under a fluorescent microscope. The rate of GFP positive cells was determined by flow cytometry.
Results
1. The acquired HBVE could retain the surface protein HBsAg + pre S1 + pre S2 and had no virus DNA. It had good package ability for pIRES2-EGFP. 2. Transfection efficiency: The GFP could be observed in both the liposome group and HBVE group under the fluorescent microscope. But the HBVE group had a higher fluorescent intensity than liposome group. The transfection rate of liposome group was 49.97% ± 2.37% while the HBVE group was 70.65% ± 3.15% and the fluorescent intensity of the HBVE group was 3–4 times (P = 0.000) for liposome group with the determination of flow cytometry. 3. Targeting ability: The GFP could be observed in the four groups under the fluorescent microscope. The HepG 2 group had the highest fluorescent intensity among the four groups. The transfection rate of HepG 2 group was 71.35% ± 0.03% which was highly expressed than other groups (P = 0.000) and the fluorescent intensity of the HepG 2 group was 2–3 times (P = 0.000) for the other 3 groups with the determination of flow cytometry.
Conclusion
HBVE can be constructed successfully with the methods of PEG8000 and β-propiolactone from the supernatant fluid of HepG 2.2.15 cells. The HBVE can be a candidate gene transfer vector for liver cancer cells.
Similar content being viewed by others
References
Park CW, Park YM, Lee GT, et al. Targeting of therapeutic gene expression to the liver by using liver-type pyruvate kinase proximal promoter and the SV40 viral enhancer active in multiple cell types. Biochem Biophys Res Commun, 2004, 314: 131–137.
Park SG, Jeong YJ, Lee YY, et al. Hepatitis B virus neutralizing anti-pre-S1 human antibody fragments from large naïve antibody phage library. Antiviral Res, 2005, 68: 109–115.
Wang LQ, Kaneko S, Honda M, et al. Approach to establishing a liver targeting gene therapeutic vector using naturally occurring defective hepatitis B viruses devoid of immunogenic T cell epitope. Virus Res, 2002, 85: 187–197.
Kasuya T, Kuroda S. Nanoparticles for human liver-specific drug and gene delivery systems: in vitro and in vivo advances. Expert Opin Drug Deliv, 2009, 6: 39–52.
Gao X, Kim KS, Liu D. Nonviral gene delivery: what we know and what is next. AAPS J, 2007, 9: E92–104.
Kaneda Y, Nakajima T, Nishikawa T, et al. Hemagglutinating virus of Japan (HVJ) envelope vector as a versatile gene delivery system. Mol Ther, 2002, 6: 219–226.
Broo K, Wei J, Marshall D, et al. Viral capsid mobility: a dynamic conduit for inactivation. PNAS, 2001, 98: 2274–2277.
Hilleman MR. Critical overview and outlook: pathogenesis, prevention, and treatment of hepatitis and hepatocarcinoma caused by hepatitis B virus. Vaccine, 2003, 21: 4626–4649.
Yamada T, Iwabuki H, Kanno T, et al. Physicochemical and immunological characterization of hepatitis B virus envelope particles exclusively consisting of the entire L (pre-S1 + pre-S2 + S) protein. Vaccine, 2001, 19: 3154–3163.
Payan C, Pivert A, Kampf G, et al. Assessment of new chemical disinfectants for HBV virucidal activity in a cell culture model. J Hosp Infect, 2004, 56: S58–63.
Maruyama K, Suzuki R, Takizawa T, et al. Drug and gene delivery by “bubble liposomes” and ultrasound. Yakugaku Zasshi (Japanese), 2007, 127: 781–787.
Zhong ZR, Liu J, Deng Y, et al. Preparation and characterization of a novel nonviral gene transfer system: procationic-liposome-protamine-DNA complexes. Drug Deliv, 2007, 14: 177–183.
Gershon H, Ghirlando R, Guttman SB, et al. Mode of formation and structural features of DNA-cationic liposome complexes used for transfection. Biochemistry, 1993, 32: 7143–7151.
Kahán Z, Csenki M, Varga Z, et al. The risk of early and late lung sequelae after conformal radiotherapy in breast cancer patients. Int J Radiat Oncol Biol Phys, 2007, 68: 673–681.
Gao YS, Mei J, Tong TL, et al. Inhibitory effects of VEGF-siRNA mediated by adenovirus on osteosarcoma-bearing nude mice. Cancer Biother Radiopharm, 2009, 24: 243–247.
Brandwijk RJ, Griffioen AW, Thijssen VL. Targeted gene-delivery strategies for angiostatic cancer treatment. Trends Mol Med, 2007, 13: 200–209.
Kuwada E, Noguchi K, Seo N, et al. A novel strategy of cancer gene therapy by transcriptional targeting of an allogeneic histocompatibility transgene. Anticancer Res, 2009, 29: 1015–1022.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by a grant from the National Natural Sciences Foundation of China (No. 30100189).
Rights and permissions
About this article
Cite this article
Pan, D., Wang, W., Wang, D. et al. Hepatitis B virus envelope as a targeting gene transfer vector for hepatic cancer cells. Chin. -Ger. J. Clin. Oncol. 8, 447–452 (2009). https://doi.org/10.1007/s10330-009-0084-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10330-009-0084-9