Abstract
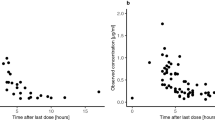
Objective: To study the cerebrospinal fluid pharmacokinetics of intravenously administered high dose-methotrexate (HD-MTX) and provide a solid fundament for clinical practice. Methods: MTX at a high dose ranging from 1.0 to 3.0 g per course was intravenously administered to 30 patients with malignant tumors. Blood and CSF samples were consecutively collected up to 36 h after the initiation of infusion (6 h). MTX concentrations were measured by using a reversed phase high-performance liquid chromatography (RP-HPLC) assay. Results: CSF MTX concentrations were (1.65±1.52)×10-6, (4.3±3.34)×10-7, (1.46±1.10)×10-7 and (3.19±4.38)×10-8 mol/L, respectively, at 0, 6, 12 and 24 h post infusion, and became undetectable at 36 h post infusion. The concentration-time curve of CSF MTX closely resembled that of the plasma MTX and fitted with the following linear regression equation: Ŷ=0.057 97+0.010 82X (Ŷ: CSF MTX concentration, X: Plasma MTX concentration, r=0.8357). Conclusion: CSF MTX was metabolized in a linear two-compartment model. Additionally, pharmacokinetic analysis of MTX levels indicated a positive correlation between CSF MTX and plasma MTX levels.
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
ZHAO, F., WANG, Q., ZHAO, Y. et al. Cerebrospinal Fluid Pharmacokinetics of Intravenous High Dose-Methotrexate. Chinese-German J Clin Oncol 5, 101–103 (2006). https://doi.org/10.1007/s10330-005-0454-x
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10330-005-0454-x