Abstract
An increasing number of plant viruses and viroids have been reported from all over the world due largely to metavirogenomics approaches with technological innovation. Herein, the official changes of virus taxonomy, including the establishment of megataxonomy and amendments of the codes of virus classification and nomenclature, recently made by the International Committee on Taxonomy of Viruses were summarized. The continued efforts of the plant virology community of Japan to index all plant viruses and viroids occurring in Japan, which represent 407 viruses, including 303 virus species and 104 unclassified viruses, and 25 viroids, including 20 species and 5 unclassified viroids, as of October 2021, were also introduced. These viruses and viroids are collectively classified into 81 genera within 26 families of 3 kingdoms (Shotokuvirae, Orthornavirae, Pararnavirae) across 2 realms (Monodnaviria and Riboviria). This review also overviewed how Japan’s plant virus/viroid studies have contributed to advance virus/viroid taxonomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Phytopathological Society of Japan (PSJ) was established in 1916 and is among the oldest phytopathological societies in the world. The PSJ or her members have contributed to a great extent to the advancement of virology since then. Examples include discoveries of many new plant viruses (see below), the first demonstration of the transmission of a plant virus by vector insects (Ando 1910) and ovarial transmission (Fukushi 1933, 1969), discovery of the oldest record of a plant virus disease (see below; Inouye and Osaki 1980), development of a protoplast system for virus research (Takebe and Otsuki, 1969), discovery of the cell-to-cell movement function of a plant virus (Nishiguchi et al. 1978) and development of a plant virus vaccine and its commercialization (Natsuaki 2011). The Plant Virus Disease Study Group (PVDSG) and Plant Virus Taxonomy Committee (PVTC) were created under the PSJ organization in 1989 and 1990, respectively. The PVDSG offers workshops with state-of-the-art talks usually every 2 years that cover related plant virus diseases from basic to applied perspectives and discuss relevant issues. The proceedings are published for every workshop (ISSN 0919-2956). The PVTC has played important roles in indexing all viruses occurring in Japan and examining and approving their Japanese and English names proposed mainly by their discoverers. The PVTC provides a list of “Plant viruses and viroids occurring in Japan” at https://www.ppsj.org/pdf/mokuroku-viroid_2021.pdf?1005 and updates it on a regular basis. This list allows the community to know whether a virus of interest has already been identified in Japan. Of over 1000 recognized plant virus/viroid species, 409 virus species are currently listed (see below). The Plant Protection Station of Japan (PPS) and the Control Station for Pests (CSP) of each prefecture are of great help in screening for newly emerged viruses in Japan. The PPS performs plant virus surveillance to determine whether imported materials (seeds or seedlings) are infected with viruses. The CSP makes tremendous efforts to survey the occurrence of plant pests, including viruses, at the field level and report on newly identified emerging diseases and pests. More than 300 reports have been published for virus emergence in the past 20 years.
Metaviromic analyses revolutionized contemporary virology and have impacted many associated areas, including studies on virus taxonomy, diversity, phylogeny and evolution, and etiology (Dolja and Koonin 2018). In particular, a vast number of virus-like sequences have increasingly been reported from various sources, such as environmental samples, insects, fungi, and plants, and many of them have been shown to belong to new taxa. Unusual viruses with as-yet-unreported genome structures have been found, and the virus taxonomy of the International Committee on Taxonomy of Viruses (ICTV) has been modified to a great extent. A few main changes made to the ICTV taxonomy include the approval of virus species based solely on the genomic sequences covering entire coding domains but lacking their terminal non-coding sequences or without the biological characterization of their members (Walker et al. 2019). Thus, putative viral sequences (metagenomes) derived from metagenomic/transcriptomic analyses can be approved by the ICTV, even without confirming their original host organisms or infectivity. “CMI/AAB Descriptions of Plant Viruses,” published by the Commonwealth Mycological Institute and The Association of Applied Biologists and is now available online https://www.dpvweb.net/, was well received as an encyclopedia of plant viruses and cited by the community. Chapters of this series provide very useful, reliable information on many properties, including the biological activity of each virus. However, sufficient information is no longer available for many species recently approved by the ICTV on the basis of metagenomic/transcriptomic analysis. An even greater change has occurred in the establishment of virus megataxonomy (Koonin et al. 2020), as exemplified by the creation of higher taxa such as “realms,” “kingdoms,” “phyla,” and “orders” (see below; Siddell et al. 2019). Lastly, the binominal “genus-species” naming system for virus species, i.e., a genus name + a species epithet, has been adopted by the ICTV, which could be (1) Genus + Latin or Latinized epithet, (2) Genus + alphanumeric epithet, or (3) Genus + freeform (Siddell et al. 2020). In the case of “Tobacco mosaic virus” for example, a change of the species to “Tobamovirus tmv,” “Tobamovirus tabaci,” “Tobamovirus mosium,” “Tobamovirus nicotianae,” or “Tobamovirus iwanowskii” will occur (Siddell et al. 2020). In the transition period, there will be a chaotic mixture of two naming systems, binominal and free form.
Given the reorganization of virus taxonomy and changes in ICTV rules, the PVDSG and PVTC decided to collaborate to prepare an article that introduces the PSJ’s contributions to ICTV taxonomy. This review provides information on what has been changed from the early virus taxonomy, what plant viruses have been reported from Japan thus far, and how virus research in Japan has contributed to virus taxonomy. Therefore, the readers are referred to some other elegant reviews for epidemiological information on plant viruses occurring in Japan (Hibi and Ohki 2015; Tsuda 2021) and appraisal of the many achievements made by the PSJ and her members (Yoshikawa et al. 2015). We would like to apologize for being unable to cite all related articles due to page limitations.
Megataxonomy of viruses recently established by the ICTV
The construction of a classification system is essential for analyzing the functions and characteristics of living organisms and studying their relationships with other species by objectively comparing and contrasting them. Viruses also have a genome and express some functions from that of their host organism; thus, the same concept can be applied. Since the discovery of tobacco mosaic virus established the concept of a “virus,” a vast number of virus species have been found in animals (vertebrates, invertebrates, and protozoa), plants (higher plants and algae), fungi, bacteria, and archaea. The ICTV was established in 1973 for the classification and nomenclature of viruses, including viroids and satellites, based on various characteristics, such as the molecular composition of the genome; the structure of the virus capsid and whether or not it is enveloped; the gene expression program used to produce virus proteins; host range; pathogenicity; and sequence similarity. However, as new viruses have been continuously discovered since then, the classification criteria have been revised at a dizzying pace almost every year.
In 2014, the 29th edition of the master species list (MSL 29) was published by the ICTV, in which viruses were classified into 7 orders, 104 families (23 subfamilies), 505 genera, and 3185 species based on the criteria of the 9th ICTV report. The following year, Hibi and Ohki (2015) published the “Encyclopedia of Plant Viruses,” which included 3 orders, 25 families (3 subfamilies), 112 genera, and 1235 species of plant and fungus viruses that had been discovered all over the world, according to the criteria of ICTV MSL29. However, the ICTV continued to subdivide the virus classification system, and ICTV MSL33 was published in 2018 with “Phylum” and “Class” being added to the previous “Order.” In ICTV MSL34, which was additionally published in the same year, “Realm,” which corresponds to the domain newly established in the taxonomic class of organisms as a result of molecular phylogenetic analysis, was placed at the top. In the 35th MSL in 2019, “Kingdom” was added between "Realm" and "Phylum" to complete the prototype of the current virus classification system. According to ICTV MSL36 issued in 2021 (https://talk.ictvonline.org/files/master-species-lists/m/msl/12314), all viruses established as species can be classified into 6 realms, 10 kingdoms, 17 phyla (2 subphyla), 39 classes, 59 orders (8 suborders), 189 families (136 subfamilies), 2224 genera (70 subgenera), and 9110 species. Among them, plant and fungus viruses and viroids, including satellites, contain 2 realms, 3 kingdoms, 7 phyla (2 subphyla), 16 classes, 19 orders, 46 families (12 subfamilies), 193 genera (5 subgenera), and 2134 species. Notably during this period, “Tolecusatellitidae,” as a new family was established in ICTV MSL31 published in 2016, for satellite nucleotides incorporated into virus particles of members of the family Geminiviridae, and the genera Betasatellite and Deltasatellite were generated directly under the family. The following year, in ICTV MSL32, the satellite nucleotides associated with viruses of the families Geminiviridae and Nanoviridae were established as the family Alphasatellitidae, containing the two subfamilies, Geminialphasatellitinae and Nanalphasatellitinae, and the subfamily Petromoalphasatellitinae was subsequently added to the family (Tsuda 2021).
However, as evidenced by the review process of the virus classification system over the past 50 years, improvements to the system are still being made at an ever-increasing pace. Detailed international information on virus classification, including the 10th ICTV report and the latest classification list, can be obtained from the ICTV website (https://talk.ictvonline.org). In the latest version of ICTV MSL36, some viruses have been renamed and others have been reassigned to new classes for classification.
The nomenclature of viruses has been under debate in the international arena since the inception of the ICTV, and the future of nomenclature remains unpredictable. For the past half century, the scientific nomenclature of viruses has been based not on the Latinized (Linnaean) binomial nomenclature for animals and plants but on the non-Latinized binomial nomenclature that reflects the parasitic nature of viruses, biological phenomena, and so on (Van Regenmortel 2019). However, some members of the ICTV executive committee suggested that viral genome databases had accumulated so much in this century that it was time to apply the Latinized binomial nomenclature (Siddell et al. 2020). A review of the nomenclature is currently being actively discussed on the ICTV website (https://talk.ictvonline.org/files/ictv_documents/m/binomial-nomenclature). As we write this manuscript, the Archives of Virology, the official journal of the ICTV, has excitingly been publishing passionate papers by taxonomists advocating the retention of the non-Latinized nomenclature or proposing the development of new nomenclature so that both virologist and non-virologist stakeholders can be well understood by each other (Gibbs 2020; Hull and Rima 2020). Virologists, as well as life scientists, should keep a close eye on this debate, as it will be relevant to them when they write their papers.
Taxonomic placement of plant viruses/viroids reported from Japan
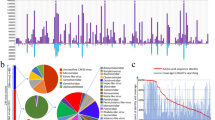
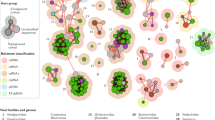
The plant viruses, viroids, and satellites reported in Japan before 2021 are summarized in Fig. 1, Table 1 and Supplementary Table S1. Many of these isolates are available from the National Agriculture and Food Research Organization (NARO) GeneBank https://www.gene.affrc.go.jp/databases-micro_pl_diseases_en.php. The viruses belong to 2 realms, 3 kingdoms, 6 phyla, 12 classes, 15 orders, 24 families, 75 genera, and 407 species, including 104 unclassified species. The viroids are classified into 2 families, 6 genera, and 25 species, including 5 unclassified species. In addition, six species of the genus Betasatellite and one unclassified satellite species have been recognized. In a decade with the diffusion of deep sequencing technologies, newly identified viruses have been increasing at a rapid pace. In fact, the Encyclopedia of Plant Viruses published in 2015 (Hibi and Ohki) listed 349 species as viruses reported in Japan. Thus, about 60 species have been newly reported in just over 5 years. Of these, 28 viruses, such as citrus leaf blotch virus, plum bark necrosis stem pitting-associated virus, and pelargonium zonate spot virus, have been found by deep sequencing (Ito and Sato 2020; Uehara-Ichiki et al. 2018; Kamitani et al. 2017). Eighteen of these viruses represent 18 novel species that had never before been reported in the world, and the remaining viruses were found for the first time to occur in Japan. Reports of virus infection in woody fruit trees, flowers, and medicinal plants are especially increasing. The PPS has exercised vigilance against all non-native viruses/viroids unlisted in the table at https://www.ppsj.org/pdf/mokuroku-viroid_2021.pdf?471005, particularly destructive ones, such as plum pox virus (PPV) and potato spindle tuber viroid (PSTVd). Viruses of the genera Ipomovirus, Tritimovirus, and others have not yet invaded Japan. Notably, sweet potato mild mottle virus (genus Ipomovirus) and wheat streak mosaic virus (genus Tritimovirus) have emerged worldwide, and their emergence in Japan is being actively avoided. In addition to the two viruses, maize chlorotic mottle virus (genus Machlomovirus), rice tungro bacilliform virus (genus Tungrovirus), sweet potato chlorotic stunt virus (genus Crinivirus), faba bean necrotic yellows virus (genus Nanovirus), broad bean stain virus (genus Comovirus), tomato brown rugose fruit virus (genus Tobamovirus), tomato mottle mosaic virus (genus Tobamovirus) and pepino mosaic virus (genus Potexvirus) are important emerging viruses in the world (Jones 2021) but have not been detected in Japan (Table 2). The PPS has also been cautious especially for pospiviroids (genus Pospiviroid), including PSTVd, columnea latent viroid, Mexican pepita viroid, pepper chat fruit viroid, tomato apical stunt viroid, tomato chlorotic dwarf viroid, and tomato planta macho viroid, because of their considerably destructive nature. Globalization and climate change can lead to unexpected viral spread. Therefore, there should be an awareness of potential agricultural pandemics caused by plant viruses and viroids.
Discoveries of plant viruses in Japan associated with the creation of new taxa
Realm Monodnaviria
Kingdom Shotokuvirae
The realm Monodnaviria consists of four kingdoms, of which only the kingdom Shotokuvirae (phylum Cressdnaviricota) contains plant viruses in two virus families: Geminiviridae (order Geplafuvirales) and Nanoviridae (order Mulpavirales). The kingdom Shotokuvirae was named after Japan’s Empress Shotoku (718–770 AD), who reigned over the country twice (as Empress Koken and later as Empress Shotoku) and left the world’s earliest written record of a plant virus disease, which depicted geminivirus infection. In the summer of 752 AD, Empress Koken visited the city of Nara and noticed a eupatorium plant that had turned conspicuously bright yellow (Fig. 2a). Her Majesty delivered a poem to followers (Fig. 2b), which was later included in Man'yoshu, a revered anthology of Japanese poetry compiled in the late eighth century. Currently, it is believed that the yellowing eupatorium plant was infected with the geminivirus eupatorium yellow vein virus (EpYVV; Inouye and Osaki 1980; Saunders et al. 2003).
a Yellow vein symptoms of the eupatorium plant. Right: a eupatorium plant (Eupatorium makinoi) infected with the geminivirus, eupatorium yellow vein virus. Left: a healthy eupatorium plant. (Courtesy of Dr. Sachiko Funayama-Noguchi, The University of Tokyo). b A monument at Saidaiji Temple, Nara Prefecture, Japan commemorating Empress Koken’s poem included in Man’yoshu. An English translation of the poem reads: “Perhaps it does frost/In this village morn by morn/For the grass I saw in the field of summertime/Has already turned yellow” (Suga, T. The Man’yo-shu: A Complete English Translation in 5–7 Rhythm, Kanda Inst. Foreign Lang., Tokyo, 1991)
Geminiviridae is the largest family of viruses containing 14 genera and 520 species, and is characterized by a non-enveloped, twinned (geminate) icosahedral virion, and the genome is composed of one or two single-stranded (ss), circular DNAs of 2.5–5.2 kb. Geminiviruses are transmitted by whiteflies, leafhoppers, or other hemipterans in a persistent, circulative, non-propagative manner. In Japan, members of eight species in the genus Begomovirus, one species in the genus Maldovirus, and one species in the genus Mastrevirus have been officially approved (Table 1 and Supplementary Table S1).
Begomoviruses are further divided into four groups: the Old World begomoviruses, the New World begomoviruses that lost the AV2 movement protein gene, “legumoviruses,” and “sweepoviruses.” Two Old World begomoviruses, EpYVV and eupatorium yellow vein mosaic virus, were first discovered and characterized in Japan (Onuki and Hanada 2000). These viruses, as well as other Old World begomoviruses in Japan, including ageratum yellow vein virus, ageratum yellow vein Hualian virus, honeysuckle yellow vein virus, and tomato yellow leaf curl virus, have a monopartite genome (Ogawa et al. 2008; Ueda et al. 2004). In contrast, Japan’s only New World begomovirus, abutilon mosaic virus, has a bipartite genome with DNA A and B, in which DNA B-encoded genes complement the functions of the lost AV2 gene (Table 1 and Supplementary Table S1). Japan’s only “sweepovirus” sweet potato leaf curl virus (SPLCV) was first discovered in Japan in 1975 and characterized later (Onuki and Hanada 1998). SPLCV and its close relatives have been reported worldwide.
The mastrevirus miscanthus streak virus, infecting a gramineous weed Miscanthus sacchariflorus, has been reported only in Japan (Yamashita et al. 1985b), while its closest phylogenetic relative, eragrostis minor streak virus, has been reported only in Namibia, Africa, infecting another grass Eragrostis minor. These observations may imply the presence of unexplored mastrevirus flora within wild grasses.
The family Nanoviridae is characterized by a multipartite, circular, ssDNA genome of 0.9–1.1 kb. The isometric virions each contain a single genome component and are transmitted by aphids in a circulative, non-propagative, persistent manner. The family consists of two genera: the genus Babuvirus contains viruses that infect monocotyledonous plants and have six genome components, and the genus Nanovirus contains viruses that primarily infect dicotyledonous plants and have eight genome components. Milk vetch dwarf virus, belonging to the genus Nanovirus, was first discovered in 1949 in Japan and later characterized (Sano et al. 1998). The virus naturally infects a wide range of legumes and some solanaceous plants in east and Southeast Asia. Another virus in the family Nanoviridae in Japan is banana bunchy top virus in the genus Babuvirus, which is a serious threat to banana production in Asia, Oceania, and Africa.
Realm Riboviria
Phylum Duplornaviricota , kingdom Orthornavirae
Order Reovirales
The order Reovirales consists only of the family Reoviridae, which includes viruses of plants, mammals, birds, fish, insects, ticks, fungi, shellfish, and clams (ICTV MSL36). They generally form multi-layered spherical particles, each containing a set of 9–12 double-stranded (ds) RNA genomic segments. The family is divided into the subfamilies Spinareovirinae (nine genera) and Sedoreovirinae (six genera), depending on the presence or absence of spikes on the inner capsid. Members of the subfamily Sedoreovirinae have turrets but not those of the subfamily Spinareovirinae. Viruses infecting land plants belong to the genera Phytoreovirus (three species) of Sedoreovirinae and Fijivirus (nine species) and Oryzavirus (two species) of Spinareovirinae. At present, the occurrence of four plant reoviruses has been reported in Japan: rice dwarf virus (RDV) in the genus Phytoreovirus, rice black streaked dwarf virus (RBDV) and southern rice black streaked dwarf virus (SRBDV) in the genus Fijivirus, and rice ragged stunt virus (RRSV) in the genus Oryzavirus (Iida et al. 1972; Shikata 1974; Supplementary Table S1). RDV and RBDV occur in various parts of Japan and overwinter in their leafhopper vectors (Isogai et al. 1995; Murao et al. 1994). In contrast, infection of SRBDV and RRSV in rice plants begins to spread by viruliferous planthoppers carried by the westerlies from southeastern Asia (Matsukura et al. 2013). The brown (Nilaparvata lugens) and white-backed planthoppers (Sogatella furcifera), which are the insect vectors for RRSV and SRBDV, respectively, cannot overwinter in Kyushu and other regions north of Kyushu, Japan.
RDV causes dwarf disease in rice crops and was first recognized in 1883 in Shiga Prefecture, Japan, and Takada and Hashimoto demonstrated the causal relationship between leafhoppers and rice dwarf disease in 1885 (Iida et al. 1972). Furthermore, Ando (Ando 1910) reported that the disease was transmitted by leafhoppers and was not sucking damage caused by the insect. This discovery has been the first in the world to report the transmission of a plant virus by a vector. Subsequently, world-famous discoveries continued. Fukushi (1933) revealed that RDV is transmitted from viruliferous leafhoppers to their progeny by transovarial transmission. Additionally, Fukushi (1939) presumed that RDV propagates in the insect vector. Finally, Fukushi et al. (1960) clearly demonstrated RDV propagation in leafhoppers by electron microscopy.
In Japan, complete nucleotide sequences of RDV genome segments were determined from 1987 to 1994 (Uyeda et al. 1987, 1994). The analysis led RDV to be the first plant reovirus to be sequenced. Thus, the function of the proteins encoded by the genome was predicted from their amino acid sequences (Suzuki 1995). However, it is still difficult to analyze gene function in relation to biological properties since reverse genetics systems for plant reoviruses have not been established. Nevertheless, Uyeda et al. (1995) succeeded in showing a method to analyze the gene function of RDV. They co-injected two RDV isolates with different biological properties into leafhoppers to reassort their genome segments. After letting the injected insects feed on rice seedlings, reassortants with different combinations of their genomic segments were isolated from the infected seedlings. These artificially created reassortments made it possible to analyze how the replacements of each genome segment affected biological properties. In addition, Kimura (1984) succeeded in establishing cultured cells of the RDV insect vector and creating inoculation methods using the cells with RDV. Using the cultured cells, Yan et al. (1996) and Tomaru et al. (1997) clearly demonstrated that the P2 protein encoded by the genome segment S2 is crucial for infection of RDV in insect cells and transmission of the virus by the insect vector.
Phylum Kitrinoviricota, kingdom Orthornavirae
Order Hepelivirales
The order Hepelivirales consists of four families, among which only the family Benyviridae accommodates plant viruses. This family has only one genus, Benyvirus, and four species.
Beet necrotic yellow vein virus (BNYVV), the exemplar member of genus Benyvirus, causes “rhizomania” disease of sugar beet worldwide. This widespread soil-borne disease severely reduces the yield of sugar beet. The causal agent of this disease was first characterized and named in Japan in the early 1970s. BNYVV has rod-shaped particles with four or five positive-sense (+) ssRNA segments (RNA1–5). The smaller genomic components (RNA3, RNA4, and RNA5) play important roles in pathogenicity (rhizomania symptoms) and plasmodiophorid (Polymyxa betae) vector transmission. BNYVV RNA3 (and probably RNA5 in some cases) is strongly associated with Rz1 resistance breaking in sugar beet cultivars. Key achievements were made in Japan that enhanced the understanding of the pathosystem (Tamada 2016; Tamada and Kondo 2013; Tamada et al. 2021). BNYVV was also found in spinach in Japan (Fujisawa et al. 1982).
Burdock mottle virus (BdMoV), another Benyvirus species, was isolated from an edible burdock plant (Arctium lappa L.) in 1970. Burdock is a root vegetable crop unique to Japan and is the only known natural host of BdMoV. The bipartite genome sequence of BdMoV is available (Kondo et al. 2013).
Order Martellivirales
Of the seven families in the order Martellivirales, the families Bromoviridae, Closteroviridae, Endornaviridae, Mayoviridae, and Virgaviridae accommodate plant viruses. Members of these families have mono- to tri-segmented (+)ssRNA genomes and form filamentous, rod-shaped, or icosahedral particles, except for capsid-less endornaviruses.
Asparagus virus 2 (AV-2) is a member of the genus Ilarvirus in the family Bromoviridae. Fujisawa et al. (1983) revealed the biological, physical, morphological, and serological properties of a Japanese strain of AV-2. Shimura et al. (2013) further revealed low genetic variability among AV-2 isolates and clarified the function of the 2b protein of AV-2 as an RNA suppressor. Subsequently, Kawamura et al. (2014) suggested the involvement of pollen transmission in co-infection by two AV-2 isolates in plants.
Cucumber yellows virus (CuYV) in the genus Crinivirus of the family Closteroviridae was first isolated from cucumber and melon plants in Japan in 1979 (Yamashita et al. 1979). Systemic yellowing was observed on the original host plants, and the greenhouse whitefly was identified as the CuYV vector. Hartono et al. (2003) determined the complete nucleotide sequence of the bipartite genome to classify CuYV as a new species in the genus Crinivirus. Thus, the characterization of CuYV contributed to the recognition of diversity of the genus Crinivirus. Furthermore, a new cucurbit-infecting virus was reported in Japan in 2009 (Gyoutoku et al. 2009). Subsequently, Okuda et al. (2010) determined the entire nucleotide sequence of this virus’ genomic RNA, and the virus was named cucurbit chlorotic yellows virus to propose as a new species in the genus Crinivirus.
The family Endornaviridae contains two genera, Alphaendornavirus and Betaendornavirus. Plants can serve as natural hosts of members of the genus Alphaendornavirus. Fukuhara et al. (1993) discovered two linear dsRNA elements (16 kb and about 13.2 kb) in Japonica rice plants (Oryza sativa L. for Oryza sativa endornavirus) and in O. rufipogon (for Oryza rufipogon endornavirus), which is an ancestor of O. sativa. Subsequently, Fukuhara et al. (2006) molecularly characterized distinct large dsRNAs from several plant species. Okada et al. (2011, 2014) proposed bell pepper endornavirus from bell pepper and Basella alba endornavirus from Malabar spinach. In addition, two high-molecular-mass dsRNAs were isolated from common bean and proposed as Phaseolus vulgaris endornavirus 1 and 2 by Okada et al. (2013). A series of these discoveries of large dsRNAs contributed to establishing the distinct species in the genus Alphaendornavirus of the family Endornaviridae.
The Mayoviridae family is composed of two relatively small genera, Idaeovirus and Pteridovirus. Among their members is the representative idaeovirus, raspberry bushy dwarf virus (RBDV), reported from Japan (Isogai et al. 2012). RBDV has a bi-partite genome (Natsuaki et al. 1991), and RNA1 encodes an RNA silencing suppressor, which is also involved in efficient lateral transmission through pollen (Isogai et al. 2019, 2020).
The family Virgaviridae accommodates seven genera, including Furovirus, Goravirus, Hordeivirus, Pecluvirus, Pomovirus, Tobamovirus, and Tobravirus. Members of this family have mono- to tri-partite, (+)ssRNA genomes with rod-shaped particles. The number of genome components varies depending upon the genus. The transmission modes provide the basis for genus demarcation; i.e., virgavirids are transmitted by plasmodiophorids or nematodes, or through pollen and/or seed. Gentian ovary ringspot virus (GORV) was discovered and was fully characterized for the first time in Japan (Atsumi et al. 2015), which led to the creation of the genus Goravirus. This virus shows asymmetrical pollen transmission in two plant species: gentian and Nicotiana benthamiana. GORV is horizontally transmissible to N. benthamiana via pollination with virus-infected pollens, whereas gentian can be infected by GORV via pollination with infected pollens only from gentian (Isogai et al. 2017). For the genus Pomovirus, broad bean necrosis virus (BBNV) was first discovered in broad bean in 1951 in Kyushu, Japan and later characterized biologically (Inouye and Asatani 1968). BBNV is a soil-borne virus, but its vector is unknown. Lu et al. (1998) determined the complete sequences of the tri-partite genome of BBNV. In the genus Furovirus, the wheat mosaic disease caused by a furovirus was first found in Japan in the 1920s. This causal virus had long been considered as a strain of soil-borne wheat mosaic virus (SBWMV) that was originally reported from the United States, but it was designated as Japanese soil-borne wheat mosaic virus (JSBWMV). Similar furoviruses also were reported from China and Europe, which were designated as Chinese wheat mosaic virus (CWMV) and European wheat mosaic virus, respectively. These four viruses are transmitted by the same plasmodiophorid vector (Polymyxa graminis) and induce the same or similar type of symptoms in wheat plants. However, there is amino acid sequence divergence (8–48%) among the proteins or domains encoded by the four viruses (Diao et al. 1999; Shirako et al. 2000), and therefore, they were classified as separate species. In Japan, SBWMV and CWMV have been isolated from Hokkaido and Tochigi Prefecture, respectively. JSBWMV has been molecularly and biologically well-characterized (Miyanishi et al. 2002; Ohsato et al. 2003; Yamamiya et al. 2005). Spontaneous deletion of the readthrough domain, downstream of the capsid protein (CP) coding domain, of JSBWMV RNA2 results in more severe symptom induction relative to the wild-type virus. As interspecies reassortment of RNA1 and RNA2 was observed between JSBWMV and SBWMV in the laboratory experiment (Miyanishi et al. 2002), mixed infections of wheat by these furoviruses may allow for the emergence of new strains (Tamada and Kondo 2013). Of members of the genus Tobamovirus, 15 viruses have been reported from Japan (Table 1 and Supplementary Table S1), among which the following tobamoviruses were first found and characterized in Japan. Kyuri green mottle mosaic virus, which was originally designated as the cucumber and Yodo strains of cucumber green mottle mosaic virus (CGMMV), occurred in cucumber plants in western Japan in 1966 (Inouye et al. 1967). The Japanese word "Kyuri" was adopted as part of the species name by Francki et al. (1986). The complete genome sequence of the virus was determined by Tan et al. (2000). Wasabi mottle virus was first isolated as a wasabi strain of tobacco mosaic virus (TMV) from wasabi plants (Eutrema wasabi) in Tochigi Prefecture, and later was characterized molecularly by Shimamoto et al. (1998). Cucumber mottle virus was first isolated from a greenhouse-grown cucumber in 1998 in Miyazaki Prefecture, and was characterized molecularly and serologically by Orita et al. (2007). This virus was approved as the exemplar strain of a new species. In Japan, sudden outbreaks of the diseases caused by tobamoviruses have often been undergone in various horticultural crops, and have raised awareness of the importance of studies not only on their etiology but also on their epidemiology and control. For some tobamoviruses, e, g., TMV, tomato mosaic virus (ToMV) and CGMMV, molecular and biological properties of attenuated viruses were well characterized (Nishiguchi and Kobayashi 2011; Nishiguchi 2017). An attenuated virus has been used for controlling the mosaic disease of tomatoes caused by ToMV in fields.
Order Tymovirales
The order Tymovirales consists of 5 families, 24 genera, and 2 subgenera, including 219 species (Fig. 1), according to ICTV MSL36 released in 2021. Compared to the 2009 version (ICTV 9th Report), one family, two subfamilies, eight genera, and two subgenera have been newly established, and 74 new species have been added. Viruses in Tymovirales have a single molecule of (+)ssRNA and are either isometric (the family Tymoviridae) or flexuous filamentous (the families Alphaflexiviridae, Betaflexiviridae and Gammaflexiviridae) in particle morphology. Here are some examples of research in Japan that have contributed to the establishment of new genera and species in the Tymovirales.
Sumi et al. (1993) partially purified filamentous virus particles from garlic plants (Allium sativa) showing mosaic symptoms and determined the 3′-terminal regions of their RNA genomes. They discovered four new viruses named garlic virus-A, B, C, and D (GVA–GVD) from diseased plants. Later, they determined the complete nucleotide sequences of GVA and GVC (Sumi et al. 1999). Yamashita et al. (1996) also detected a flexuous filamentous virus with a length of 700–800 nm in mosaic-diseased garlic plants and showed that the virus was transmitted by the eriophyid mite, Aceria tulipae. They reported the virus as garlic mite-borne mosaic virus, which is currently a synonym for GVC. From these pioneer studies, the genus Allexivirus was established as a mite-borne filamentous virus with a different genome organization from viruses of the genus Potexvirus (garlic mosaic virus) in the family Alfaflexiviridae and the genus Carlavirus (garlic latent virus) in the family Betaflexiviridae (subfamily Quinvirinae).
There is a graft-transmissible disease in apples called “topworking disease” that occurs only in Japan. The causal viruses were found to be apple chlorotic leaf spot virus (ACLSV), apple stem grooving virus (ASGV), and apple stem pitting virus (ASPV; Yanase 1974), which are currently the exemplar members of the genera Trichovirus, Capillovirus, and Foveavirus, respectively, of the Betaflexiviridae family. Though these viruses generally infect commercial apple cultivars without symptoms, the viruses cause disease in apple trees grown on wild apple rootstocks (e.g., Malus prunifolia, M. sieboldii) in Japan. Koganezawa and Yanase (1990) purified an elongated virus that was 800 nm long from Nicotiana occidentalis plants infected with ASPV. The properties of RNAs and proteins of ACLSV and ASGV from diseased apples were analyzed by Yoshikawa and Takahashi (1988), and later complete nucleotide sequences of the genomes of ACLSV and ASGV from apples were determined (Sato et al. 1993; Yoshikawa et al. 1992). Citrus tatter leaf virus was found to be the same virus as ASGV (Yoshikawa et al. 1993). Recently, apple russet ring and apple green crinkle, graft-transmitted diseases first reported more than 60 years ago, were shown to be caused by ACLSV and ASPV variants, respectively (Li et al. 2020). In addition, a Foveavirus (cherry virus B), which appeared to be a new species, was detected in sweet cherry (Prunus avium) in Japan (Yaegashi et al. 2020).
Order Tolivirales
Member viruses of the order Tolivirales infect insects but mostly plants. The name of the virus order “Toli” is a syllabic abbreviation of “tombus-like.” Most of the members belong to the family Tombusviridae, except for one virus species in the genus Alphacarmotetravirus in the family Carmotetraviridae. The family Tombusviridae contains three subfamilies, Calvusvirinae, Procedovirinae, and Regressovirinae (Fig. 1), and one subfamily-unassigned genus (Luteovirus). Viruses in the genus Luteovirus were originally classified into the former family Luteoviridae, while the other three genera Enamovirus, Polerovirus, and Sobemovirus in the former family Luteoviridae were recently reclassified into the new family Solemoviridae in the order Sobelivirales (ICTV MSL36). The subfamily Calvusvirinae contains only one genus (Umbravirus). The subfamily Procedovirinae contains 14 genera and 6 genus-unassigned species. Of the 14 genera, isolated in Japan are viruses of 6 genera, Alphacarmovirus, Alphanecrovirus, Betacarmovirus, Betanecrovirus, Gammacarmovirus and Tombusvirus. The subfamily Regressovirinae contains only one genus (Dianthovirus), but no dianthovirus has been isolated in Japan. Virions of the members in the order Tolivirales are spherical and have an icosahedral capsid without an envelope, except viruses in the genus Umbravirus, which lack a coat protein open reading frame (ORF) but are encapsidated with a helper virus. All viruses in the order Tolivirales have a monopartite, (+)ssRNA genome, except viruses in the genus Dianthovirus in the subfamily Regressovirinae, which uniquely have a bipartite genome.
Within member viruses classified into the order Tolivirales, 18 viruses have been reported to be found in Japan. Among those viruses, the following toliviruses were first discovered in Japan. Melon necrotic spot virus was discovered in 1960 (Kishi 1966) and assigned as the representative member of the genus Gammacarmovirus (ICTV MSL36). Pea stem necrosis virus (genus Gammacarmovirus) was discovered in 1976 (Osaki et al. 1988; Suzuki et al. 2002). Japanese iris necrotic ring virus (genus Betacarmovirus) was discovered in 1982 (Takemoto et al. 2000). Adonis mosaic virus (genus Alphacarmovirus) was discovered in 2017 (Yasaki et al. 2017). Soybean dwarf virus (SbDV, genus Luteovirus) was discovered in 1968 and characterized (Tamada et al. 1969; Terauchi et al. 2001). Subterranean clover red leaf virus was proposed to belong to a new virus species in 1982 but later merged into SbDV in 1991 (ICTV MSL12). Recently, two tombusviruses were isolated from Japanese gentian plants cultivated in northeastern Japan. A novel tombusvirus was isolated and tentatively named gentian virus A (Fujisaki et al. 2018). Another tombusvirus, Sikte waterborne virus strain C1, was also isolated from Japanese gentian, and both tombusvirus isolates have been shown to have unique properties, namely host-plant specific low-temperature-dependent multiplication abilities (Fujisaki et al. 2020, 2021). A taxonomically interesting plant virus, gentian Kobu-sho-associated virus (GKaV), was characterized from gentian plants with stunting symptoms in Iwate Prefecture, Japan (Kobayashi et al. 2013). The virus was reported to have a long undivided dsRNA genome of 23.3 kb with a single large ORF (Kobayashi et al. 2013). However, the helicase and/or RdRP (RNA-dependent RNA polymerase) domain of GKaV shows phylogenetic affinity to animal flaviviruses with (+)ssRNA genomes. GKaV has not yet been assigned to any taxon by the ICTV and may represent a new species in a distinct class or order within the phylum Kitrinoviricota.
Phylum Negarnaviricota, kingdom Orthornavirae
Order Serpentovirales
The order Serpentovirales consists of one family, Aspiviridae (former Ophioviridae), one genus, Ophiovirus, and seven species (Table 1 and Supplementary Table S1). Ophioviruses infect only plants, and citrus psorosis virus (CPsV) (Ito et al. 2011) and Mirafiori lettuce big-vein virus (MiLBVV) are present worldwide and cause serious problems for citrus and lettuce production, respectively. MiLBVV, lettuce ring necrosis virus, tulip mild mottle mosaic virus (TMMMV), and freesia sneak virus (FreMV) are transmitted by soil-inhabiting fungi of the genus Olpidium. However, the vectors of CPsV, blueberry mosaic associated virus (BlMaV), and ranunculus white mottle virus are unknown. The genome of ophioviruses consists of three or four individually encapsidated negative-sense (–) and possibly ambi-sense ssRNA segments.
Mild mottle mosaic disease induces serious quantitative and qualitative production losses in the tulips grown in Japan. The causal agent of the diseases was thought to be a virus, but the entity was unknown. In 1995, Morikawa et al. (1995) discovered its virions as thin and circular filamentous particles about 3 nm in diameter with different contour lengths, adopting open and linear conformations. Around the same time, a similar virion was discovered in a psorosis disease of citrus, a worldwide problem, the causal agent of which had, however, been unknown for almost a hundred years (Garcia et al. 1994). The causal agent of the mild mottle mosaic diseases of tulip (TMMMV) was thought to be an ophiovirus (Morikawa et al. 1995), and later, its partial genomic sequence was confirmed via RT-PCR (Vaira et al. 2003). Along with TMMMV, four other ophioviruses (MiLBVV, CPsV, FreMV, and BlMaV) have been reported in Japan (Isogai et al. 2016; Ito et al. 2011; Natsuaki et al. 2002).
Ophiovirus virion morphology resembles that of the viruses in the genus Tenuivirus, family Phenuiviridae, and the internal nucleocapsid component of ophiovirus resembles that of members of the family Tospoviridae. However, ophioviruses do not infect plants in the Gramineae like the tenuiviruses and do not have enveloped virions, as do members of the family Tospoviridae. Ophioviruses do not have the conserved identical nucleotides at the genomic RNA termini that are typical of these two families. Phylogenetic trees using amino acid sequences conserved the core modules of RdRPs in ophioviruses, and representative (−)ssRNA viruses reinforce the separation of ophioviruses from other (−)ssRNA viruses (Naum-Onganía et al. 2003; van der Wilk et al. 2002). The family Ophioviridae was proposed to the ICTV 9th Report, and only one family and one genus, Ophiovirus, were recognized, but the order was not assigned (King et al. 2011). Presently, the family has been renamed Aspiviridae and assigned to higher taxa: realm Riboviria, phylum Negarnaviricota, subphylum Haploviricotina, class Milneviricetes, and order Serpentovirales (Garcia et al. 2017; ICTV MSL36).
Order Mononegavirales
While many members of the family Rhabdoviridae in the order Mononegavirales infect animals, some are known to infect plants. Plant rhabdoviruses have basically enveloped virions with an unsegmented (−)ssRNA genome and are transmitted in a persistent and propagative manner by leafhoppers, planthoppers, or aphids (Dietzgen et al. 2020). They were originally separated into two genera, Cytorhabdovirus and Nucleorhabdovirus, based on their morphogenesis sites and molecular phylogenies. The latter genus was recently divided into three new genera: Alphanucleorhabdovirus, Betanucleorhabdovirus, and Gammanucleorhabdovirus (ICTV MSL36).
Two cereal rhabdoviruses, northern cereal mosaic virus (cytorhabdovirus) and rice yellow stunt virus (synonymous with rice transitory yellowing virus, an alphanucleorhabdovirus), were first discovered in Japan in 1944 and Taiwan in 1960, respectively, and their Japanese isolates have been well characterized biologically and molecularly (Hiraguri et al. 2010; Tanno et al. 2000). Another novel plant rhabdovirus, namely persimmon virus A, a cytorhabdovirus, was discovered in Japanese persimmon trees with fruit apex disorder via deep-sequencing analysis (Ito et al. 2013). Several unassigned plant rhabdoviruses or rhabdovirus-like agents have also been historically recorded in various monocot and dicot diseased plants, including butterbur, burdock, broad bean, carrot, Colmanara, gloriosa, elder, Epiphyllum, Euonymus, lotus, strawberry, tomato, and wasabi, along with a known cytorhabdovirus, strawberry crinkle virus (Table 1 and Supplementary Table S1).
Another important insight into plant rhabdoviral research in Japan was the discovery of bi-segmented rhabdoviruses for unique cases in the order Mononegavirales (Kondo et al. 2003, 2006; Sasaya et al. 2004). Orchid fleck virus (OFV) and a tobacco strain, formerly known as tobacco stunt virus, of lettuce big vein-associated virus (LBVaV) were first found in Japan in 1969 and 1950, respectively. Two bipartite rhabdoviruses share several characteristics with classical plant rhabdoviruses, but their virions, short bacilliform for OFV and rod shapes for LBVaV, appear not to be enveloped. OFV is transmitted by false spider mites (Brevipalpus californicus) in a persistent manner, while LBVaV is transmitted by zoospores of the chytrid fungus (O. virulentus) (Kondo et al. 2003; Sasaya et al. 2001). OFV has been found to naturally infect citrus trees in Mexico, and one orchid strain potentially originated as a result of a reassortment event (Dietzgen et al. 2018; Kondo et al. 2017). These discoveries led to the creation of two novel genera, Dichorhavirus and Varicosavirus, with OFV and LBVaV, respectively, as the exemplar strains within the family Rhabdoviridae (ICTV MSL36). Currently, all six genera for plant rhabdoviruses, including the two genera, Dichorhavirus and Varicosavirus, have been classified within a newly created subfamily Betarhabdovirinae. Notably, a new varicosavirus and a new emaravirus (fimovirid, see below) were recently detected from Vitis coignetiae (crimson glory vine) in Japan and likely represent new species (Nabeshima and Abe 2021).
Order Bunyavirales
The order Bunyavirales contains 12 families, 54 genera, and 477 species (ICTV MSL36). While the majority of bunyaviruses infect animals, plant-infecting members of the families Tospoviridae (genus Orthotospovirus), Phenuiviridae (genera Tenuivirus, Laulavirus, and Rubodvirus), and Fimoviridae (genus Emaravirus) are rapidly expanding (Kormelink et al. 2021).
Among 26 ICTV-recognized species of Tospoviridae, seven of their exemplar viruses have been recorded in Japan. Melon yellow spot virus (MYSV) was identified as a distinct orthotospovirus from melon in Shizuoka Prefecture in 1992 (Kato et al. 2000). MYSV has become one of the most devastating pathogens in cucumber cultivation and was correlated with the recent decrease in insecticide sensitivity of the vector Thrips palmi (Takagi et al. 2018). Another cucurbit orthotospovirus, watermelon silver mottle virus, was first identified in Okinawa Prefecture in 1982 (Iwaki et al. 1984). Capsicum chlorosis virus (CaCV), which sometimes occurs in pepper fields in Japan with a limited incidence rate. T. palmi was identified as a vector of CaCV with low transmissibility (Chiaki et al. 2020). The other orthotospoviruses that invaded and have spread throughout Japan include tomato spotted wilt virus, chrysanthemum stem necrosis virus, impatiens necrotic spot virus, and iris yellow spot virus (Doi et al. 2003; Inouye and Inouye 1972; Tanina et al. 2001; Supplementary Table S1). A putative orthotospovirus, lisianthus necrotic spot virus, has only been reported in Japan (Shimomoto et al. 2014).
Discovery and genome identification of rice stripe virus (RSV) and rice grassy stunt virus (RGSV) led to the establishment of the cognate species and the genus Tenuivirus in family Phenuiviridae (Toriyama 1982; Toriyama et al. 1997). An epidemic of RGSV was recorded in the 1970–1980s in Kyushu (Hibino 1985; Iwasaki and Shinkai 1979). RSV greatly affected rice production from the 1900s to the 1960s. In the vector leafhopper, Laodelphax striatella, RSV proliferates and is transovarially transmitted (Fukushi 1986). Today, in Japan, a number of rice cultivars harboring the RSV resistance gene Stvb-i are widely used. Recently, Hayano-Saito and Hayashi (2020) identified Stvb-i as a large protein with a domain homologous to the histidine kinase/HSP-90-like ATPase superfamily. A new phenuivirid was recently discovered from tulip that appears to represent a new species (Neriya et al. 2021).
As the sole genus in the family Fimoviridae, Emaravirus was established in 2009 and has expanded from 11 recognized species in 2020–2021 in 2021 (ICTV MSL36). About three-fourths of them infect woody plants, while the rest infect herbaceous plants. Some emaraviruses have been shown to be transmitted by eriophyid mites. In Japan, the occurrence of six emaraviruses has been recognized. Fig mosaic virus, transmitted by Aceria ficus, was first found in Shimane Prefecture (Ishikawa et al. 2012) and has since been reported in several prefectures. Perilla mosaic virus (PerMV) and Japanese star anise ringspot-associated virus (JSARaV) were first identified from cultivated herb (Shiso, Perilla frutescens) and Japanese star anise (Shikimi, Illicium anisatum), respectively, in Kochi Prefecture (Kubota et al. 2020; Shimomoto et al. 2022). PerMV and JSARaV have been shown to be transmitted by the eriophyid mite, Shevtchenkella sp., and the distinct eriophyid mite, a member of the family Diptilomiopidae, respectively. Pear chlorotic leaf spot-associated virus (PCLSaV) was first reported in Asian pear varieties in China (Liu et al. 2020). PCLSaV was also found in Japan from European and Japanese pear varieties, and Eriophyes chibaensis Kadono is suspected as a vector for PCLSaV (Kubota et al. 2021a). Chrysanthemum mosaic-associated virus was discovered in Aichi Prefecture from chrysanthemum leaves affected by ringspot symptoms called “Mom-mon” disease (Kubota et al. 2021b, c).
Phylum Pisuviricota, kingdom Orthornavira
Order Durnavirales
The order Durnavirales contains dsRNA and (+)ssRNA viruses from five families: Amalgaviridae, Cyrvulaviridae, Hypoviridae, Partitiviridae, and Picobirnaviridae. The Partitiviridae is a family of five genera, Alphapartitivirus, Betapartitivirus, Cryspovirus, Deltapartitivirus, and Gammapartitivirus, and unassigned species. The hosts are different depending on the genus, such as plants and fungi for Alphapartitivirus and Betapartitivirus, protozoa for Cryspovirus, plants for Deltapartitivirus, and fungi for Gammapartitivirus.
Small virus-like particles (VLPs) were found in apparently healthy Fabaceae plants, white clover, and alfalfa, from Italy and Japan in the early 1980s (Boccardo et al. 1983; Natsuaki et al. 1984). They were found to possess dsRNA in their VLPs and were therefore named white clover cryptic virus 1, 2, and 3 (WCCV1, 2 and 3) and alfalfa cryptic virus 1 (ACV1). The complete nucleotide sequences of WCCV1 and WCCV2 were determined in Italy in 2005 and in Germany in 2013, respectively (Boccardo and Candress 2005; Lesker et al. 2013). These studies indicated a distant relationship between plants infecting partitiviruses; therefore, WCCV1 was classified as a type species of the genus Alphapartitivirus and WCCV2 as a species of the genus Betapartitivirus. Since the molecular status of WCCV3 and ACV1 are unclear, they are unassigned species in the family Partitiviridae.
Several dsRNA molecules have been found in Japanese pear (Osaki et al. 1998). Three of them were transmitted through both ovules and pollen, not by grafting. Sequencing analysis revealed that one dsRNA encodes RdRP and the other two encode two CP variants similar to viruses in the genus Deltapartitivirus, and it was thereby named Pyrus pyrifolia partitivirus 1 (Osaki et al. 1998, 2017). Two other dsRNA molecules from Japanese pear were sequenced and found to encode RdRP and CP, both of which were similar to viruses in the genus Alphapartitivirus (Osaki and Sasaki 2018). These two dsRNAs were named Pyrus pyrifolia partitivirus 2. Moreover, several dsRNA viruses in the family Partitiviridae were isolated in Japan. A seedborne small spherical virus was isolated from seedlings of Japanese radish with yellow edge symptoms and named the radish yellow edge virus (Natsuaki et al. 1979). RYEV was reported to have two CP variants and a few segmented dsRNAs (Natsuaki et al. 1983). Virus particles found in healthy carrots were found to include several dsRNAs, and RNA-RNA hybridization analysis indicated the existence of four dsRNA viruses, named carrot temperate virus-1 to -4 (Natsuaki et al. 1990). A seedborne cryptic virus was isolated from spinach in Japan and named spinach temperate virus and was reported to have three segmented dsRNA genomes (Natsuaki et al. 1983). However, molecular characterization was not performed; these viruses are still unassigned members of the family Partitiviridae.
Order Picornavirales
The order Picornavirales contains eight families: Caliciviridae, Dicistroviridae, Iflaviridae, Marnaviridae, Picornaviridae, Polycipiviridae, Secoviridae, and Solinviviridae. Members of the order infect a wide range of hosts, including humans, animals, plants, and microorganisms, and all plant viruses in the order are only included in the family Secoviridae. Members of the family Secoviridae share common properties with the other members of the order: (1) they are non-enveloped spherical viruses with mono- or bipartite linear (+)ssRNA genomes that may have a small viral protein termed VPg at the 5′-termini; (2) they produce large polyproteins that are cleaved by viral proteases, and (3) the coat protein consists of one to three components, depending on the genus. The attachment of VPg to the genomic RNA has not yet been explicitly shown for many members, and experimental verification is needed.
The family Secoviridae includes the genera Cheravirus, Sadwavirus, Sequivirus, Torradovirus, and Waikavirus, which do not belong to any subfamily, and the three genera Comovirus, Fabavirus, and Nepovirus in the subfamily Comovirinae.
Apple latent spherical virus (ALSV) was the first member to be fully sequenced in the genus Cheravirus (Li et al. 2000). ALSV has been widely utilized as a versatile virus vector in diverse plants for various purposes, including stable expression of foreign gene products, virus-induced gene silencing, and plant virus vaccination (Igarashi et al. 2009; Satoh et al. 2014). ALSV was also the first plant virus with three capsid proteins in which the 3D structure was determined at the atomic level (Naitow et al. 2020).
The genus Waikavirus is named after the Japanese word “Waika,” meaning stunting, a symptom associated with rice disease (RTD). Rice tungro spherical virus (RTSV), one of the two major virus players involved in RTD, is the exemplar strain of the representative species of the genus (Hibino 1996). Another player, rice tungro bacilliform virus (a DNA tungrovirus), is the major contributor to symptom induction, while its transmission depends on RTSV.
The genus Sadwavirus, which includes the species Satsuma dwarf virus, was created after molecular characterization, and phylogenetic analysis revealed that satsuma dwarf virus (SDV) is distinct from viruses in other known genera (Iwanami et al. 1999; Le Gall et al. 2007). Satsuma, often synonymous with Kagoshima Prefecture, is a geographical name in Japan that is presumed to be the provenance of Citrus unshiu (satsuma). SDV shares many properties with members of the family Secoviridae; the spherical virions have a CP that consists of two components, and they separately contain bipartite linear (+)ssRNA genomes. The gene organization of RNA1 and RNA2 is also similar to that of the other members of the family Secoviridae. However, only poor phylogenetic relationships have been found among SDV and the other secovirids. In particular, the amino acid sequence between the conserved catalytic cysteine (or serine) of the 3C-like protease and the GDD motif of the RdRP has been used to distinguish SDV from other secovirids (Iwanami et al. 1999; Sanfaçon et al. 2020).
SDV is widely spread in major citrus-producing areas in Japan (Iwanami 2010) and also occurs in Korea, China, and Turkey. SDV infects most citrus cultivars and citrus relatives (Iwanami 2010). Most typically, infected trees develop smaller boat- or spoon-shaped leaves with short internodes in satsuma mandarin (Citrus unshiu). After several years, both tree vigor and fruit quality are reduced. Boat- or spoon-shaped leaves are not induced on orange (Citrus × sinensis), tangor, or other hybrid cultivars, but leaves become smaller and narrower. As the disease progresses, tree vigor and fruit quality are also affected (Iwanami 2010; Kato et al. 2019).
Recently, the reorganization of the genus Sadwavirus to create three new subgenera was proposed; the proposed subgenera were “Satsumavirus” (including SDV), “Stramovirus” (including strawberry mottle virus, SMoV), and “Cholivirus” (Sanfaçon et al. 2020). SDV and SMoV commonly occur in Japan, whereas other viruses that belong to the genus Sadwavirus have not been reported. A few distinct SDV strains were recognized, and their taxonomic status deserves reconsideration (Ito et al. 2004; Iwanami 2010; Iwanami et al. 2001; Yan et al. 2021).
Order Sobelivirales
The order Sobelivirales contains (+)ssRNA viruses from three families: Alvernaviridae, Barnaviridae, and Solemoviridae. While the family Alvernaviridae and Barnaviridae includes an algal virus and a fungal virus, respectively, the family Solemoviridae includes viruses infecting plants. The family Solemoviridae is a recently created family that includes viruses from genera Sobemovirus, Polemovirus, Enamovirus, and Polerovirus and unassigned species. The two genera Enamovirus and Polerovirus and the unassigned species were previously classified into the former Luteoviridae but reclassified into the family Solemoviridae. Enamo- and poleroviruses are transmitted in a circulative, non-propagative, persistent manner by specific aphid vectors.
Leaf roll disease is one of the most serious potato diseases, first recognized in 1916, but the causal agent, potato leafroll virus (PLRV), was also mentioned in 1920 in Japan, followed by a series of studies characterizing it in Japan (Kojima 1974). Although the genome of PLRV was originally supposed to be dsDNA (Sarker 1976), it was discovered in Japan that the genomes of PLRV and another polero-like virus, tobacco necrotic dwarf virus (TNDV), which was first reported in Japan in 1977, were ssRNAs (Takanami and Kubo 1979). Since sequence analysis was performed on Japanese isolates of PLRV (Ohshima et al. 1993) but not on TNDV, TNDV is still an unassigned member of the family Solemoviridae.
In the genus Polerovirus, pepper yellows disease (PYD) caused by pepper vein yellows virus (PeVYV) was found in bell pepper plants grown in Okinawa Prefecture, Japan, and the causal virus was isolated (Yonaha et al. 1995). The complete nucleotide sequence of the virus was determined and found to be a new species of the genus Polerovirus (Murakami et al. 2011). After that, PYD was reported around the world, including in Israel, China, Australia, Spain, and Saudi Arabia. Molecular characterization revealed that these viruses are closely related but distinct species in the genus Polerovirus; therefore, PeVYV-1 to -6 was proposed for their names (Fiallo-Olivé et al. 2018; Kamran et al. 2018). Yellows disease of sugar beet, which was first recorded in Hokkaido in the 1960s, was thought to be caused by beet western yellows virus (BWYV), which is known to induce yellowing diseases of many crops worldwide. However, Yoshida and Tamada (2019) recently identified the causal virus as a new virus, beet leaf yellowing virus (BLYV), which appears to belong to a new species of the genus Polerovirus. In addition, brassica-infecting BWYV-like virus was identified as brassica yellows virus (BrYV) (high affinity with turnip yellows virus). BLYV and BrYV are widely distributed in Asia but not in Western countries. Both viruses are not yet classified into formal species.
In the genus Sobemovirus, two viruses were isolated and characterized in Japan. Ryegrass mottle virus was isolated from Italian ryegrass and cocksfoot with mottling and necrotic symptoms in Japan (Toriyama et al. 1983). The genome sequence was characterized and shown to be a new species of the genus Sobemovirus (Zhang et al. 2001). Cymbidium chlorotic mosaic virus was isolated from spring orchid causing stunting and chlorotic streaks in Japan in 1996 (Kondo et al. 1996). Molecular analysis of the virus revealed that it was a new monocot-infecting member of the genus Sobemovirus (Kondo et al. 2015).
Order Patatavirales
All members of the family Potyviridae, which is the only family in the order Patatavirales, infect plants. The virions of the potyvirids are non-enveloped and flexuous filamentous particles with an unsegmented or segmented (+)ssRNA genome(s). The genomes have a VPg (viral protein genome-linked) covalently attached to the 5′-end, and the 3′-terminus is polyadenylated. The genomes encode a major ORF, which is translated into a large polyprotein (Gibbs and Ohshima 2010) and a small overlapping ORF, a “pretty interesting Potyviridae ORF, PIPO” (Chung et al. 2008). The large polyprotein is autocatalytically hydrolyzed into at least ten functional proteins.
The members of the family Potyviridae are separated into 12 genera: Arepavirus, Bevemovirus, Brambyvirus, Bymovirus, Celavirus, Ipomovirus, Macluravirus, Poacevirus, Potyvirus, Roymovirus, Rymovirus, and Tritimovirus, based on their biological characteristics, genome structures, nucleotide sequence identities, and molecular phylogenies (Wylie et al. 2017). While the members of the genera Potyvirirus and Macluravirus are transmitted in a non-persistent manner by aphids, members of other genera are transmitted by whiteflies (ipomoviruses), eriophyid mites (rymoviruses, poaceviruses, and tritimoviruses), or the plasmodiophorid cercozoan Polymyxa graminis (bymoviruses). The vectors of members of the two remaining genera, Roymovirus and Brambivirus, are unknown. Some members of the family Potyviridae are seedborne (Gibbs and Ohshima 2010).
Two hundred thirty-five species in the family Potyviridae have been reported (https://talk.ictvonline.org/ictv-reports/ictv_online_report/positive-sense-rna-viruses/w/potyviridae; June 2021). Potyviruses constitute one of the largest genera of plant viruses, and approximately 190 species belong to the genus Potyvirus. The largest number of nucleotide sequences of full or nearly full genomes of the potyviruses in GenBank are probably turnip mosaic virus (TuMV; > 600 isolates; Kawakubo et al. 2021), potato virus Y (PVY; > 600 isolates; Gao et al. 2020), and PPV (Maejima et al. 2020; < 500 isolates; June 2021).
The oldest description of symptoms caused by a virus in the family Potyviridae in Japan seems likely to be mosaic symptoms on cowpea (Matsumoto 1922), and the casual agents of this symptom seem to be one of the potyviruses, e.g., bean common mosaic virus. The members in five genera, Bymovirus, Macluravirus, Potyvirus, Roymovirus, and Rymovirus, have been reported in Japan (2019): five bymoviruses, barley mild mosaic virus (Kashiwazaki 1996), barley yellow mosaic virus (Kashiwazaki et al. 1990), rice necrosis mosaic virus (Wagh et al. 2016), wheat yellow mosaic virus (Namba et al. 1998), soybean leaf rugose mosaic virus (Ohki et al. 2021); two macluraviruses, Chinese yam necrotic mosaic virus (ChyNMV; Kondo and Fujita 2012) and narcissus latent virus (Ohshima et al. 2016); one roymovirus member, rose yellow mosaic virus (RoYMV; Ohata et al. 2021); one rymovirus, ryegrass mosaic virus (Supplementary Table S1); and 68 potyviruses. The five bymoviruses, ChyNMV and RoYMV were discovered or molecularly characterized in Japan for the first time. Among the 68 potyviruses are those discovered in Japan for the first time and representing new species. Examples include ashitaba mosaic virus (Sakamoto et al. 2021), Japanese yam mosaic virus (Fuji and Nakamae 1999), Habenaria mosaic virus (Kondo et al. 2014) and konjak mosaic virus (Nishiguchi et al. 2006), uraria mosaic virus (Nakasato et al. 2020), and passiflora mosaic virus Y (Nakasato et al. 2020).
Phylum Artverviricota, kingdom Pararnavirae
Order Ortervirales
The order Ortervirales consists of five families: Retroviridae, Belpaoviridae, Metaviridae, Pseudoviridae, and Caulimoviridae. The first comprises human and animal pathogenic classical retroviruses. The following three families include retrotransposons found in diverse organisms, including plants, fungi, and invertebrates. Although studies of retrotransposons have contributed to the progress of plant biology (e.g., functional genomics; for review, see Hirochika 2001), this section covers plant pathogenic members classified into the family Caulimoviridae, with a focus on viruses detected for the first time in Japan and mostly assigned to species. Caulimoviridae consists of 11 genera, such as Badnavirus, Caulimovirus, Petuvirus, and Soymovirus (ICTV MSL36). Among all of their members, viruses of ten species, six from the genus Caulimovirus, two from the genus Soymovirus, and one each from the genera Petuvirus and Badnavirus, have been reported in Japan. In addition, virus strains belonging to two tentative species of the genus Badnavirus have been suggested in the list of “Plant Viruses and Viroids in Japan” (https://www.ppsj.org/pdf/mokuroku-viroid_2021.pdf?1005; Table 1 and Supplementary Table S1).
The first description of the cauliflower mosaic virus (CaMV, genus Caulimovirus, family Caulimoviridae) in Japan was in 1960 (Tochihara 1960). To date, nine CaMV isolates have been described and recently sequenced (Yasaka et al. 2014), of which five strains are available from the NARO gene bank (https://www.ppsj.org/pdf/mokuroku-viroid_2021.pdf?1005). The incidence of carnation etched ring virus (CERV) was described only briefly (Tochihara et al. 1975), and following that, there has been no report of CERV in Japan. Dahlia mosaic virus, which was described with virion morphology in 1967, has been frequently found in surveys of dahlia plants and included as a target in a multiplex PCR for virus disease diagnosis in dahlia (Asano et al. 2015). The strawberry vein banding virus was described to a minor extent in a report of a long-term study on different strawberry viruses (Takai 1973). Its virion structure was electron-microscopically confirmed later, and the genome was recently sequenced (Takamura et al. 2020).
Soybean chlorotic mottle virus, the exemplar member of the genus Soymovirus, was identified for the first time in Japan (Iwaki et al. 1984) as representing a new virus species. The genome sequence and functional analysis have also been reported (Hasegawa et al. 1989; Takemoto and Hibi 2001). Another member of the genus Soymovirus found in Japan is the blueberry red ringspot virus, which has been detected in at least two distinct regions of Japan (Isogai et al. 2009).
Petunia vein clearing virus (PVCV) was first identified in the United States (Lesemann and Casper 1973) and reported in Japan for the first time in 1980 (Supplementary Table S1). PVCV is the best-studied Caulimoviridae member with the virus genome integrated into host chromosomes (Richert-Pöggeler et al. 2003). A recent study revealed that the activation of endogenous PVCV affects floral color patterning, likely by silencing its suppressor function (Kuriyama et al. 2020).
Canna yellow mottle virus (CYMV) is a member of the genus Badnavirus discovered in Japan (Yamashita et al. 1985a). It was identified based on similar morphological features to the cacao swollen shoot virus. Although CYMV was first observed in Japan, it has rarely been described in Japan. In contrast, CYMV has been reported in different countries, including the United States (Zhang et al. 2017). Aucuba ringspot virus, tentatively representing a new species of the genus Badnavirus, was reported to have molecular and morphological properties similar to other members of the genus (Uke et al. 2021). However, its identity with the Aucuba bacilliform virus, which is listed as a related, unclassified virus by the ICTV, remains to be determined.
Viroids
Families Avsunviroidae and Pospiviroidae
Viroids are small, circular, and non-protein-coding ssRNAs that replicate autonomously through an RNA-RNA rolling-circle mechanism mediated by host enzymes. Currently, 33 species have been identified, which are classified into three genera (Avsunviroid, Elaviroid, and Pelamoviroid) of the Avsunviroidae family and five genera (Apscaviroid, Cocadviroid, Coleviroid, Hostuviroid, and Pospiviroid) of the Pospiviroidae family (Di Serio et al. 2018, 2021; Serra et al. 2018). Two species of the genus Pelamoviroid in Avsunviroidae and 18 species belonging to the five genera in Pospiviroidae have been identified in Japan (Table 1 and Supplementary Table S1).
Hop stunt viroid (HSVd), the type member of the genus Hostuviroid, was first identified in Japan as a causal agent of stunt disease in commercial hops that exhibit bine stunting and the low α-acid content in cones (Sasaki and Shikata 1977). Hop stunt disease emerged for the first time in the 1940‒1950s and was endemic only in Japan until the end of the twentieth century. However, outbreaks are now reported in the world's main hop producing areas (Hataya et al. 2017). In hosts other than hops, pale fruit disease that occurred in cucumber plants in the Netherlands was revealed to be caused by a variant of HSVd, and subsequently, HSVd variants were also detected in grapevine and citrus trees worldwide. In addition, another HSVd variant was revealed to cause the dapple fruit disease of plum and peach or the yellow fruit disease of ‘Soldam’ plum in Japan (Hataya et al. 2017). Evolutionary analyses suggested that HSVd variants in hops were transferred by chance and originated from HSVd variants in cultivated grapevines that were symptomless reservoirs, and the HSVd sequence was evolved to adapt in hops (Kawaguchi-Ito et al. 2009). Currently, HSVd is known to have a broad host range and is widely distributed around the world in plants of many families, such as Anacardiaceae (pistachio), Cannabaceae (hop), Cucurbitaceae (cucumber), Lythraceae (pomegranate), Moraceae (fig and mulberry), Rhamnaceae (jujube), Rosaceae (e.g., almond, apple, apricot, cherry, peach, pear, and plum), Rutaceae (Citrus spp.), and Vitaceae (grapevine; Hataya et al. 2017).
The genus Apscaviroid is the largest genus, consisting of ten species. The representative member, apple scar skin viroid (ASSVd), was first identified in Japan as a causal agent of scar skin or dapple apple diseases. Symptoms of ASSVd vary among apple cultivars (Koganezawa 1985). ASSVd also induces fruit dimple disease in Japanese pear cultivars ‘Niitaka’ and ‘Yoshino’ and rusty skin or fruit crinkle disease on pear cultivars in China. ASSVd was also identified in apricot, peach, sweet cherry, and Himalayan wild cherry, whose symptoms caused by ASSVd are unknown. The natural host range of ASSVd is restricted to pome and stone fruit trees in the Rosaceae family (Hadidi et al. 2017). Furthermore, the genus Apscaviroid includes citrus viroid VI, which was identified first in a tree of ‘Shiranui’ mandarin in Japan (Ito et al. 2001) and subsequently in Japanese persimmon (Nakaune and Nakano 2008).
Conclusions
This review was devised to provide the readership with information about the viruses and viroids occurring in Japan compiled by the two PSJ committees, PVDSG and PVTC, as well as the contributions made by PSJ and its previous and current members. This review cannot cover all of their achievements, and thus, the readership is recommended to refer to other elegant reviews and books (see “Introduction”). The two committees plan to periodically publish such a review article with advances in plant virus/viroid hunting and their studies in Japan.
References
Ando K (1910) About rice dwarf disease (in Japanese). J Agric Soc Jpn 34:1–3
Asano S, Matsushita Y, Hirayama Y, Naka T (2015) Simultaneous detection of Tomato spotted wilt virus, Dahlia mosaic virus and Chrysanthemum stunt viroid by multiplex RT-PCR in dahlias and their distribution in Japanese dahlias. Lett Appl Microbiol 61:113–120
Atsumi G, Tomita R, Yamashita T, Sekine KT (2015) A novel virus transmitted through pollination causes ring-spot disease on gentian (Gentiana triflora) ovaries. J Gen Virol 96:431–439
Boccardo G, Candresse T (2005) Complete sequence of the RNA1 of an isolate of White clover cryptic virus 1, type species of the genus Alphacryptovirus. Arch Virol 150:399–402
Boccardo G, Lisa V, Milne RG (1983) Cryptic viruses in plants. In: Compans RW, Bishop DHL (eds) Double-stranded RNA viruses. Elsevier, New York, pp 425–430
Chiaki Y, Kubota K, Tomitaka Y, Usugi T, Sakurai T (2020) Transmission of capsicum chlorosis virus by Thrips palmi (Thysanoptera: Thripidae). Appl Entomol Zool 55:31–35
Chung BYW, Miller WA, Atkins JF, Firth AE (2008) An overlapping essential gene in the Potyviridae. Proc Natl Acad Sci USA 105:5897–5902
Di Serio F, Li SF, Matoušek J, Owens RA, Pallás V, Randles JW, Sano T, Verhoeven JTJ, Vidalakis G, Flores R, ICTV Report C (2018) ICTV virus taxonomy profile: Avsunviroidae. J Gen Virol 99:611–612
Di Serio F, Owens RA, Li SF, Matoušek J, Pallás V, Randles JW, Sano T, Verhoeven JTJ, Vidalakis G, Flores R, ICTV Report C (2021) ICTV virus taxonomy profile: Pospiviroidae. J Gen Virol 102:001543
Diao A, Chen J, Ye R, Zheng T, Yu S, Antoniw JF, Adams MJ (1999) Complete sequence and genome properties of Chinese wheat mosaic virus, a new furovirus from China. J Gen Virol 80:1141–1145
Dietzgen RG, Freitas-Astúa J, Chabi-Jesus C, Ramos-González PL, Goodin MM, Kondo H, Tassi AD, Kitajima EW (2018) Dichorhaviruses in their host plants and mite vectors. Adv Virus Res 102:119–148
Dietzgen RG, Bejerman NE, Goodin MM, Higgins CM, Huot OB, Kondo H, Martin KM, Whitfield AE (2020) Diversity and epidemiology of plant rhabdoviruses. Virus Res 281:197942
Doi M, Zen S, Okuda M, Nakamura H, Kato K, Hanada K (2003) Leaf necrosis disease of lisianthus (Eustoma grandiflorum) caused by Iris yellow spot virus (in Japanese with English summary). Jpn J Phytopathol 69:181–188
Dolja VV, Koonin EV (2018) Metagenomics reshapes the concepts of RNA virus evolution by revealing extensive horizontal virus transfer. Virus Res 244:36–52
Fiallo-Olivé E, Navas-Hermosilla E, Ferro CG, Zerbini FM, Navas-Castillo J (2018) Evidence for a complex of emergent poleroviruses affecting pepper worldwide. Arch Virol 163:1171–1178
Francki RIB, Hu J, Palukaitis P (1986) Taxonomy of cucurbit-infecting tobamoviruses as determined by serological and molecular hybridization analyses. Intervirology 26:156–163
Fuji S, Nakamae H (1999) Complete nucleotide sequence of the genomic RNA of a Japanese yam mosaic virus, a new potyvirus in Japan. Arch Virol 144:231–240
Fujisaki K, Tateda C, Shirakawa A, Iwai M, Abe Y (2018) Identification and characterization of a tombusvirus isolated from Japanese gentian. Arch Virol 163:2477–2483
Fujisaki K, Abe Y, Tateda C, Iwai M, Kaido M, Mise K (2020) Host specific preference for low temperature in the multiplication of a tombusvirus, gentian virus A. Virus Res 286:198048
Fujisaki K, Tateda C, Abe Y, Dominguez JJA, Iwai M, Obara K, Nakamura T, Iwadate Y, Kaido M, Mise K (2021) Infectious in vitro transcripts from a cDNA clone of a Japanese gentian isolate of Sikte waterborne virus, which shows host-specific low-temperature-dependent replication. Arch Virol 166:1991–1997
Fujisawa I, Tsuchizaki T, Iizuka N (1982) Bean yellow mosaic virus, tobacco mosaic virus and beet necrotic yellow vein virus isolated from spinach (in Japanese with English summary). Ann Phytopathol Soc Jpn 48:592–599
Fujisawa I, Goto T, Tsuchizaki T, Iizuka N (1983) Some properties of asparagus virus II isolated from Asparagus officinalis in Japan. Ann Phytopathol Soc Jpn 49:683–688
Fukuhara T, Moriyama H, Pak JY, Hyakutake H, Nitta T (1993) Enigmatic double-stranded RNA in Japonica rice. Plant Mol Biol 21:1121–1130
Fukuhara T, Koga R, Aoki N, Yuki C, Yamamoto N, Oyama N, Udagawa T, Horiuchi H, Miyazaki S, Higashi Y, Takeshita M, Ikeda K, Arakawa M, Matsumoto N, Moriyama H (2006) The wide distribution of endornaviruses, large double-stranded RNA replicons with plasmid-like properties. Arch Virol 151:995–1002
Fukushi T (1933) Transmission of the virus through the eggs of an insect vector. Proc Imp Acad Jpn 9:457–460
Fukushi T (1939) Retention of virus by its insect vectors through several generations. Proc Imp Acad Jpn 15:142–145
Fukushi T (1969) Relationships between propagative rice viruses and their vectors. In: Maramorosch K (ed) Viruses, vectors, and vegetation. Wiley Interscience, New York, pp 279–301
Fukushi T (1986) Virus diseases of plants. Yokendo Co., Ltd., Tokyo
Fukushi T, Shikata E, Kimura I, Nemoto M (1960) Electron microscopic studies on the rice dwarf virus. Proc Jpn Acad 36:352–357
Gao F, Kawakubo S, Ho SYW, Ohshima K (2020) The evolutionary history and global spatio-temporal dynamics of potato virus Y. Virus Evol 6:veaa056
Garcia ML, Bó ED, Grau O, Milne RG (1994) The closely related citrus ringspot and citrus psorosis viruses have particles of novel filamentous morphology. J Gen Virol 75:3585–3590
Garcia ML, Bó ED, da Graça JV, Gago-Zachert S, Hammond J, Moreno P, Natsuaki T, Pallás V, Navarro JA, Reyes CA, Luna GR, Sasaya T, Tzanetakis IE, Vaira AM, Verbeek M, ICTV Report C (2017) ICTV Virus taxonomy profile: Ophioviridae. J Gen Virol 98:1161–1162
Gibbs A (2020) Binomial nomenclature for virus species: a long view. Arch Virol 165:3079–3083
Gibbs A, Ohshima K (2010) Potyviruses and the digital revolution. Annu Rev Phytopathol 48:205–223
Gyoutoku Y, Okazaki S, Furuta A, Etoh T, Mizobe M, Kuno K, Hayashida S, Okuda M (2009) Chlorotic yellows disease of melon caused by Cucurbit chlorotic yellows virus, a new crinivirus (in Japanese with English summary). Jpn J Phytopathol 75:109–111
Hadidi A, Barba M, Hong N, Hallan V (2017) Apple scar skin viroid. In: Hadidi A, Flores R, Randles JW, Palukaitis P (eds) Viroids and satellites. Academic Press, London, pp 217–228
Hartono S, Natsuaki T, Genda Y, Okuda S (2003) Nucleotide sequence and genome organization of Cucumber yellows virus, a member of the genus Crinivirus. J Gen Virol 84:1007–1012
Hasegawa A, Verver J, Shimada A, Saito M, Goldbach R, Van Kammen A, Miki K, Kameya-Iwaki M, Hibi T (1989) The complete sequence of soybean chlorotic mottle virus DNA and the identification of a novel promoter. Nucleic Acids Res 17:9993–10013
Hataya T, Tsushima T, Sano T (2017) Hop stunt viroid. In: Hadidi A, Flores R, Randles JW, Palukaitis P (eds) Viroids and satellites. Academic Press, London, pp 199–210
Hayano-Saito Y, Hayashi K (2020) Stvb-i, a rice gene conferring durable resistance to rice stripe virus, protects plant growth from heat stress. Front Plant Sci 11:519
Hibi T, Ohki S (2015) The encyclopedia of plant viruses. In: Hibi T, Ohki S (eds) The encyclopedia of plant viruses (in Japanese). Asakura Publishing, Tokyo
Hibino H (1985) Rice grassy stunt virus. Trop Agric Res Ser 19:165–172
Hibino H (1996) Biology and epidemiology of rice viruses. Annu Rev Phytopathol 34:249–274
Hiraguri A, Hibino H, Hayashi T, Shimizu T, Uehara-Ichiki T, Omura T, Sasaya T (2010) Complete sequence analysis of rice transitory yellowing virus and its comparison to rice yellow stunt virus. Arch Virol 155:243–245
Hirochika H (2001) Contribution of the Tos17 retrotransposon to rice functional genomics. Curr Opin Plant Biol 4:118–122
Hull R, Rima B (2020) Virus taxonomy and classification: naming of virus species. Arch Virol 165:2733–2736
Igarashi A, Yamagata K, Sugai T, Takahashi Y, Sugawara E, Tamura A, Yaegashi H, Yamagishi N, Takahashi T, Isogai M, Takahashi H, Yoshikawa N (2009) Apple latent spherical virus vectors for reliable and effective virus-induced gene silencing among a broad range of plants including tobacco, tomato, Arabidopsis thaliana, cucurbits, and legumes. Virology 386:407–416
Iida T, Shinkai A, Kimura I (1972) Rice dwarf virus. CMI/AAB descriptions of plant viruses no. 102
Inouye T, Asatani M (1968) Broadbean necrosis virus (in Japanese with English summary). Ann Phytopathol Soc Jpn 34:317–322
Inouye T, Inouye N (1972) Tomato spotted wilt virus on dahlia (in Japanese). Nogaku Kenkyu 54:79–90
Inouye T, Osaki T (1980) The first record in the literature of the possible plant virus disease that appeared in “Manyoshu”, a Japanese classic anthology, as far back as the time of 8th century (in Japanese with English summary). Ann Phytopathol Soc Jpn 46:49–50
Inouye T, Inouye N, Asatani M, Mitsuhata K (1967) Studies on cucumber green mottle mosaic virus in Japan (in Japanese). Nogaku Kenkyu 51:175–186
Ishikawa K, Maejima K, Nagashima S, Sawamura N, Takinami Y, Komatsu K, Hashimoto M, Yamaji Y, Yamamoto J, Namba S (2012) First report of fig mosaic virus infecting common fig (Ficus carica) in Japan. J Gen Plant Pathol 78:136–139
Isogai M, Uyeda I, Kimura I (1995) The genomic diversity of field isolates of rice black-streaked dwarf Fijivirus. Ann Phytopathol Soc Jpn 61:575–577
Isogai M, Ishii K, Umemoto S, Watanabe M, Yoshikawa N (2009) First report of blueberry red ringspot disease caused by Blueberry red ringspot virus in Japan. J Gen Plant Pathol 75:140–143
Isogai M, Yoshida M, Imanishi H, Yoshikawa N (2012) First report of raspberry yellows disease caused by raspberry bushy dwarf virus in Japan. J Gen Plant Pathol 78:360–363
Isogai M, Matsuhashi Y, Suzuki K, Yashima S, Watanabe M, Yoshikawa N (2016) Occurrence of blueberry mosaic associated virus in highbush blueberry trees with blueberry mosaic disease in Japan. J Gen Plant Pathol 82:177–179
Isogai M, Kamata Y, Ando S, Kamata M, Shirakawa A, Sekine KT, Yoshikawa N (2017) Horizontal pollen transmission of Gentian ovary ring-spot virus is initiated during penetration of the stigma and style by infected pollen tubes. Virology 503:6–11
Isogai M, Matsudaira T, Ito M, Yoshikawa N (2019) The 1b gene of raspberry bushy dwarf virus is a virulence component that facilitates systemic virus infection in plants. Virology 526:222–230
Isogai M, Matsudaira T, Miyoshi K, Shimura T, Torii S, Yoshikawa N (2020) The raspberry bushy dwarf virus 1b gene enables pollen grains to function efficiently in horizontal pollen transmission. Virology 542:28–33
Ito T, Sato A (2020) Three novel viruses detected from Japanese persimmon “Reigyoku” associated with graft-transmissible stunt. Eur J Plant Pathol 158:163–175
Ito T, Ieki H, Ozaki K, Ito T (2001) Characterization of a new citrus viroid species tentatively termed Citrus viroid OS. Arch Virol 146:975–982
Ito T, Iwanami T, Ieki H, Shimomura K, Shimizu S, Ito T (2004) A new virus related to Satsuma dwarf virus: the nucleotide sequence of the 3′-terminal regions of Hyuganatsu virus RNAs 1 and 2. Arch Virol 149:1459–1465
Ito T, Furuta T, Namba N (2011) First report of Citrus psorosis virus in Japan. J Gen Plant Pathol 77:257–259
Ito T, Suzaki K, Nakano M (2013) Genetic characterization of novel putative rhabdovirus and dsRNA virus from Japanese persimmon. J Gen Virol 94:1917–1921
Iwaki M, Honda Y, Hanada K, Tochihara H, Yonaha T, Hokama K, Yokoyama T (1984) Silver mottle disease of watermelon caused by tomato spotted wilt virus. Plant Dis 68:1006–1008
Iwanami T (2010) Properties and control of satsuma dwarf virus. Jpn Agr Res Q 44:1–6
Iwanami T, Kondo Y, Karasev AV (1999) Nucleotide sequences and taxonomy of satsuma dwarf virus. J Gen Virol 80:793–797
Iwanami T, Kondo Y, Kobayashi M, Han SS, Karasev AV (2001) Sequence diversity and interrelationships among isolates of satsuma dwarf-related viruses. Arch Virol 146:807–813
Iwasaki M, Shinkai A (1979) Occurrence of rice grassy stunt disease in Kyushu, Japan (in Japanese with English summary). Ann Phytopathol Soc Jpn 45:741–744
Jones RAC (2021) Global plant virus disease pandemics and epidemics. Plants 10:233
Kamitani M, Nagano AJ, Honjo MN, Kudoh H (2017) First report of Pelargonium zonate spot virus from wild Brassicaceae plants in Japan. J Gen Plant Pathol 83:329–332
Kamran A, Lotos L, Amer MA, Al-Saleh MA, Alshahwan IM, Shakeel MT, Ahmad MH, Umar M, Katis NI (2018) Characterization of pepper leafroll chlorosis virus, a new polerovirus causing yellowing disease of bell pepper in Saudi Arabia. Plant Dis 102:318–326
Kashiwazaki S (1996) The complete nucleotide sequence and genome organization of barley mild mosaic virus (Na1 strain). Arch Virol 141:2077–2089
Kashiwazaki S, Minobe Y, Omura T, Hibino H (1990) Nucleotide sequence of barley yellow mosaic virus RNA 1: a close evolutionary relationship with potyviruses. J Gen Virol 71:2781–2790
Kato K, Hanada K, Kameya-Iwaki M (2000) Melon yellow spot virus: a distinct species of the genus Tospovirus isolated from melon. Phytopathology 90:422–426
Kato M, Kageyama C, Ishii K (2019) Effects of satsuma dwarf virus infection on medium-late-maturing citrus ‘Setoka’, ‘Tamami’ and ‘Harumi’ (in Japanese with English summary). Jpn J Phytopathol 85:116–119
Kawaguchi-Ito Y, Li SF, Tagawa M, Araki H, Goshono M, Yamamoto S, Tanaka M, Narita M, Tanaka K, Liu SX, Shikata E, Sano T (2009) Cultivated grapevines represent a symptomless reservoir for the transmission of hop stunt viroid to hop crops: 15 years of evolutionary analysis. PLoS ONE 4:e8386
Kawakubo S, Gao F, Li S, Tan Z, Huang YK, Adkar-Purushothama CR, Gurikar C, Maneechoat P, Chiemsombat P, Aye SS, Furuya N, Shevchenko O, Špak J, Škoríc D, Ho SYW, Ohshima K (2021) Genomic analysis of the brassica pathogen turnip mosaic potyvirus reveals its spread along the former trade routes of the Silk Road. Proc Natl Acad Sci USA 118:e2021221118
Kawamura R, Shimura H, Mochizuki T, Ohki ST, Masuta C (2014) Pollen transmission of asparagus virus 2 (AV-2) may facilitate mixed infection by two AV-2 isolates in asparagus plants. Phytopathology 104:1001–1006
Kimura I (1984) Establishment of new cell lines from leafhopper vector and inoculation of its cell monolayers with rice dwarf virus. Proc Jpn Acad Ser B 60:198–201
King AMQ, Adams MJ, Carstens EB, Lefkowits EJ (2011) Virus taxonomy: ninth report of the International Committee on Taxonomy of Viruses. Elsevier, Academic Press, New York
Kishi K (1966) Necrotic spot of melon, a new virus disease (in Japanese with English summary). Ann Phytopathol Soc Jpn 32:138–144
Kobayashi K, Atsumi G, Iwadate Y, Tomita R, Chiba K, Akasaka S, Nishihara M, Takahashi H, Yamaoka N, Nishiguchi M, Sekine K-T (2013) Gentian Kobu-sho-associated virus: a tentative, novel double-stranded RNA virus that is relevant to gentian Kobu-sho syndrome. J Gen Plant Pathol 79:56–63
Koganezawa H (1985) Transmission to apple seedlings of a low molecular weight RNA extracted from apple scar skin diseased trees. Ann Phytopathol Soc Jpn 51:176–182
Koganezawa H, Yanase H (1990) A new type of elongated virus isolated from apple trees containing the stem pitting agent. Plant Dis 74:610–614
Kojima M (1974) Studies on potato leaf-roll virus. J Fac Agric Hokkaido Univ 57:368–430
Kondo T, Fujita T (2012) Complete nucleotide sequence and construction of an infectious clone of Chinese yam necrotic mosaic virus suggest that macluraviruses have the smallest genome among members of the family Potyviridae. Arch Virol 157:2299–2307
Kondo H, Maeda T, Mitsuhata K, Inouye N (1996) Detection of the viruses occurring in oriental Cymbidium in Japan (in Japanese with English summary). Bull Res Inst Bioresour Okayama Univ 4:149–162
Kondo H, Maeda T, Tamada T (2003) Orchid fleck virus: Brevipalpus californicus mite transmission, biological properties and genome structure. Exp Appl Acarol 30:215–223
Kondo H, Maeda T, Shirako Y, Tamada T (2006) Orchid fleck virus is a rhabdovirus with an unusual bipartite genome. J Gen Virol 87:2413–2421
Kondo H, Hirano S, Chiba S, Andika IB, Hirai M, Maeda T, Tamada T (2013) Characterization of burdock mottle virus, a novel member of the genus Benyvirus, and the identification of benyvirus-related sequences in the plant and insect genomes. Virus Res 177:75–86
Kondo H, Maeda T, Gara IW, Chiba S, Maruyama K, Tamada T, Suzuki N (2014) Complete genome sequence of Habenaria mosaic virus, a new potyvirus infecting a terrestrial orchid (Habenaria radiata) in Japan. Arch Virol 159:163–166
Kondo H, Takemoto S, Maruyama K, Chiba S, Andika IB, Suzuki N (2015) Cymbidium chlorotic mosaic virus, a new sobemovirus isolated from a spring orchid (Cymbidium goeringii) in Japan. Arch Virol 160:2099–2104
Kondo H, Hirota K, Maruyama K, Andika IB, Suzuki N (2017) A possible occurrence of genome reassortment among bipartite rhabdoviruses. Virology 508:18–25
Koonin EV, Dolja VV, Krupovic M, Varsani A, Wolf, YI, Yutin N, Zerbini FM, Kuhn JH (2020) Global organization and proposed megataxonomy of the virus world. Microbiol Mol Biol Rev 84:e00061-19
Kormelink R, Verchot J, Tao X, Desbiez C (2021) The bunyavirales: the plant-infecting counterparts. Viruses 13:842
Kubota K, Usugi T, Tomitaka Y, Shimomoto Y, Takeuchi S, Kadono F, Yanagisawa H, Chiaki Y, Tsuda S (2020) Perilla mosaic virus is a highly divergent emaravirus transmitted by Shevtchenkella sp. (Acari: Eriophyidae). Phytopathology 110:1352–1361
Kubota K, Chiaki Y, Yanagisawa H, Takeyama S, Suzuki R, Kohyama M, Horikawa T, Toda S, Kadono F (2021a) First report of pear chlorotic leaf spot-associated virus on Japanese and European pears in Japan and its detection from an eriophyid mite. Plant Dis 105:1234
Kubota K, Chiaki Y, Yanagisawa H, Yamasaki J, Horikawa H, Tsunekawa K, Morita Y (2021b) Novel degenerate primer sets for the detection and identification of emaraviruses reveal new chrysanthemum species. J Virol Methods 288:113992
Kubota K, Yanagisawa H, Chiaki Y, Yamasaki J, Horikawa H, Tsunekawa K, Morita Y, Kadono F (2021c) Complete nucleotide sequence of chrysanthemum mosaic-associated virus, a novel emaravirus infecting chrysanthemum. Arch Virol 166:1241–1245
Kuriyama K, Tabara M, Moriyama H, Kanazawa A, Koiwa H, Takahashi H, Fukuhara T (2020) Disturbance of floral colour pattern by activation of an endogenous pararetrovirus, petunia vein clearing virus, in aged petunia plants. Plant J 103:497–511
Le Gall O, Sanfaçon H, Ikegami M, Iwanami T, Jones T, Karasev A, Lehto K, Wellink J, Wetzel T, Yoshikawa N (2007) Cheravirus and Sadwavirus: two unassigned genera of plant positive-sense single-stranded RNA viruses formerly considered atypical members of the genus Nepovirus (family Comoviridae). Arch Virol 152:1767–1774
Lesemann D, Casper R (1973) Electron microscopy of petunia vein-clearing virus, an isometric plant virus associated with specific inclusions in petunia cells. Phytopathology 63:1118
Lesker T, Rabenstein F, Maiss E (2013) Molecular characterization of five betacryptoviruses infecting four clover species and dill. Arch Virol 158:1943–1952
Li C, Yoshikawa N, Takahashi T, Ito T, Yoshida K, Koganezawa H (2000) Nucleotide sequence and genome organization of Apple latent spherical virus: a new virus classified into the family Comoviridae. J Gen Virol 81:541–547
Li C, Yaegashi H, Kishigami R, Kawakubo A, Yamagishi N, Ito T, Yoshikawa N (2020) Apple russet ring and apple green crinkle diseases: fulfillment of Koch’s postulates by virome analysis, amplification of full-length cDNA of viral genomes, in vitro transcription of infectious viral RNAs, and reproduction of symptoms on fruits of apple trees inoculated with viral RNAs. Front Microbiol 11:1627
Liu H, Wang G, Yang Z, Wang Y, Zhang Z, Li L, Waqas M, Hong N, Liu H, Wang G, Hong N, Hong J, Zhang J, Xu L, Qi L (2020) Identification and characterization of a pear chlorotic leaf spot-associated virus, a novel emaravirus associated with a severe disease of pear trees in China. Plant Dis 104:2786–2798
Lu X, Yamamoto S, Tanaka M, Hibi H, Namba S (1998) The genome organization of the broad bean necrosis virus (BBNV). Arch Virol 143:1335–1348
Maejima K, Hashimoto M, Hagiwara-Komoda Y, Miyazaki A, Nishikawa M, Tokuda R, Kumita K, Maruyama N, Namba S, Yamaji Y (2020) Intra-strain biological and epidemiological characterization of plum pox virus. Mol Plant Pathol 21:475–488
Matsukura K, Towata T, Sakai J, Onuki M, Okuda M, Matsumura M (2013) Dynamics of Southern rice black-streaked dwarf virus in rice and implication for virus acquisition. Phytopathology 103:509–512
Matsumoto T (1922) Some experiments with azuki-bean mosaic. Phytopathology 12:295–297
Miyanishi M, Roh SH, Yamamiya A, Ohsato S, Shirako Y (2002) Reassortment between genetically distinct Japanese and US strains of soil-borne wheat mosaic virus: RNA1 from a Japanese strain and RNA2 from a US strain make a pseudorecombinant virus. Arch Virol 147:1141–1153
Morikawa T, Nomura Y, Yamamoto T, Natsuaki T (1995) Partial characterization of virus-like particles associated with tulip mild mottle mosaic. Ann Phytopathol Soc Jpn 61:578–581
Murakami R, Nakashima N, Hinomoto N, Kawano S, Toyosato T (2011) The genome sequence of pepper vein yellows virus (family Luteoviridae, genus Polerovirus). Arch Virol 156:921–923
Murao K, Suda N, Uyeda I, Isogai M, Suga H, Yamada N, Kimura I, Shikata E (1994) Genomic heterogeneity of rice dwarf phytoreovirus field isolates and nucleotide sequences of variants of genome segment 12. J Gen Virol 75:1843–1848
Nabeshima T, Abe J (2021) High-throughput sequencing indicates novel varicosavirus, emaravirus, and deltapartitivirus infections in Vitis coignetiae. Viruses 13:827
Naitow H, Hamaguchi T, Maki-Yonekura S, Isogai M, Yoshikawa N, Yonekura K (2020) Apple latent spherical virus structure with stable capsid frame supports quasi-stable protrusions expediting genome release. Commun Biol 3:488
Nakasato K, Fujioka S, Sugawara Y, Ono T, Nishio T, Tsuda S (2020) First detection of two potyviruses, uraria mosaic virus and passiflora mosaic virus Y, from passionfruit in Japan. J Gen Plant Pathol 86:401–404
Nakaune R, Nakano M (2008) Identification of a new Apscaviroid from Japanese persimmon. Arch Virol 153:969–972
Namba S, Kashiwazaki S, Lu X, Tamura M, Tsuchizaki T (1998) Complete nucleotide sequence of wheat yellow mosaic bymovirus genomic RNAs. Arch Virol 143:631–643
Natsuaki T (2011) Studies on molecular mechanisms of viral attenuation and cross protection. J Gen Plant Pathol 77:354–357
Natsuaki T, Yamashita S, Doi Y, Yora K (1979) Radish yellow edge virus, a seed-borne small spherical virus newly recognized in Japanese radish (Raphanus sativus L.) (in Japanese with English summary). Jpn J Phytopathol 45:313–320
Natsuaki T, Yamashita S, Doi Y, Okuda S, Teranaka M (1983) Two seed-borne double-stranded RNA viruses, beet temperate virus and spinach temperate virus. Ann Phytopathol Soc Jpn 49:709–712
Natsuaki KT, Natsuaki T, Okuda S, Teranaka M, Yamashita S, Doi Y (1984) Two seed-borne viruses from leguminous plants [Vicia faba, Medicago sativa, Trifolium repens], alfalfa temperate virus (ATV) and white clover temperate virus (WCTV). J Agric Sci Tokyo Nogyo Daigaku 29:49–55
Natsuaki T, Muroi Y, Okuda S, Teranaka M (1990) Carrot temperate cryptoviruses and RNA–RNA hybridization among their double-stranded RNA segments. Ann Phytopathol Soc Jpn 56:354–358
Natsuaki T, Mayo MA, Jolly CA, Murant AF (1991) Nucleotide sequence of raspberry bushy dwarf virus RNA-2: a bicistronic component of a bipartite genome. J Gen Virol 72:2183–2189
Natsuaki KT, Morikawa T, Natsuaki T, Okuda S (2002) Mirafiori lettuce virus detected from lettuce with big vein symptoms in Japan (in Japanese with English summary). Jpn J Phytopathol 68:309–312
Naum-Onganía G, Gago-Zachert S, Peña E, Grau O, Garcia ML (2003) Citrus psorosis virus RNA 1 is of negative polarity and potentially encodes in its complementary strand a 24K protein of unknown function and 280K putative RNA dependent RNA polymerase. Virus Res 96:49–61
Neriya Y, Morikawa T, Hamamoto K, Noguchi K, Kobayashi T, Suzuki T, Nishigawa H, Natsuaki T (2021) Characterization of tulip streak virus, a novel virus associated with the family Phenuiviridae. J Gen Virol 102:001525
Nishiguchi M (2017) Control of plant virus diseases by attenuated viruses: from past and present to future (in Japanese). Nihon Nouyaku-Gakkaishi 42:326–333
Nishiguchi M, Kobayashi K (2011) Attenuated plant viruses: preventing virus diseases and understanding the molecular mechanism. J Gen Plant Pathol 77:221–229
Nishiguchi M, Motoyoshi F, Oshima N (1978) Behaviour of a temperature sensitive strain of tobacco mosaic virus in tomato leaves and protoplasts. J Gen Virol 39:53–61
Nishiguchi M, Yamasaki S, Lu XZ, Shimoyama A, Hanada K, Sonoda S, Shimono M, Sakai J, Mikoshiba Y, Fujisawa I (2006) Konjak mosaic virus: the complete nucleotide sequence of the genomic RNA and its comparison with other potyviruses. Arch Virol 151:1643–1650
Ogawa T, Sharma P, Ikegami M (2008) The begomoviruses Honeysuckle yellow vein mosaic virus and Tobacco leaf curl Japan virus with DNAβ satellites cause yellow dwarf disease of tomato. Virus Res 137:235–244
Ohata Y, Nishio T, Tsuda S (2021) First isolation of rose yellow mosaic virus in Japan. J Gen Plant Pathol 87:295–299
Ohki T, Kuroda T, Sayama M, Maoka T (2021) Complete nucleotide sequence of soybean leaf rugose mosaic virus, an atypical member of the genus Bymovirus. Arch Virol 166:1885–1892
Ohsato S, Miyanishi M, Shirako Y (2003) The optimal temperature for RNA replication in cells infected by soil-borne wheat mosaic virus is 17 °C. J Gen Virol 84:995–1000
Ohshima K, Nakaya T, Matsumura T, Shikata E, Kimura I (1993) Nucleotide sequences of coat protein and 17K protein genes for a potato leafroll virus Japanese isolate. Ann Phytopathol Soc Jpn 59:204–208
Ohshima K, Nomiyama R, Mitoma S, Honda Y, Yasaka R, Tomimura K (2016) Evolutionary rates and genetic diversities of mixed potyviruses in Narcissus. Infect Genet Evol 45:213–223
Okada R, Kiyota E, Sabanadzovic S, Moriyama H, Fukuhara T, Saha P, Roossinck MJ, Severin A, Valverde RA (2011) Bell pepper endornavirus: molecular and biological properties, and occurrence in the genus Capsicum. J Gen Virol 92:2664–2673
Okada R, Yong CK, Valverde RA, Sabanadzovic S, Aoki N, Hotate S, Kiyota E, Moriyama H, Fukuhara T (2013) Molecular characterization of two evolutionarily distinct endornaviruses co-infecting common bean (Phaseolus vulgaris). J Gen Virol 94:220–229
Okada R, Kiyota E, Moriyama H, Toshiyuki F, Valverde RA (2014) A new endornavirus species infecting Malabar spinach (Basella alba L.). Arch Virol 159:807–809
Okuda M, Okazaki S, Yamasaki S, Okuda S, Sugiyama M (2010) Host range and complete genome sequence of Cucurbit chlorotic yellows virus, a new member of the genus Crinivirus. Phytopathology 100:560–566
Onuki M, Hanada K (1998) PCR amplification and partial nucleotide sequences of three dicot-infecting geminiviruses occuring in Japan. Ann Phytopathol Soc Jpn 64:116–120
Onuki M, Hanada K (2000) Genomic structure of a geminivirus in the genus Begomovirus from yellow vein-affected Eupatoriurn makinoi. J Gen Plant Pathol 66:176–181
Orita H, Sakai JI, Kubota K, Okuda M, Tanaka Y, Hanada K, Imamura Y, Nishiguchi M, Karasev AV, Miyata SI, Iwanami T (2007) Molecular and serological characterization of Cucumber mottle virus, a new cucurbit-infecting tobamo-like virus. Plant Dis 91:1574–1578
Osaki H, Sasaki A (2018) A novel alphapartitivirus detected in Japanese pear. Virus Genes 54:149–154
Osaki T, Ozaki K, Inouye T (1988) Some properties of pea stem necrosis virus isolated from pea in Japan (in Japanese with English summary). Ann Phytopath Soc Jpn 54:210–216
Osaki H, Sato Y, Kudo A (1998) Seed- and pollen-transmitted double-stranded RNAs detected from Japanese pear. Ann Phytopathol Soc Jpn 64:110–115
Osaki H, Sasaki A, Nakazono-Nagaoka E, Ota N, Nakaune R (2017) Genome segments encoding capsid protein-like variants of Pyrus pyrifolia cryptic virus. Virus Res 240:64–68
Richert-Pöggeler KR, Noreen F, Schwarzacher T, Harper G, Hohn T (2003) Induction of infectious petunia vein clearing (pararetro) virus from endogenous provirus in petunia. EMBO J 22:4836–4845
Sakamoto A, Kanagawa A, Kubota M, Hoshi H, Natsuaki KT (2021) Identification of a new potyvirus isolated from Angelica keiskei. J Gen Plant Pathol 87:87–93
Sanfaçon H, Dasgupta I, Fuchs M, Karasev AV, Petrzik K, Thompson JR, Tzanetakis I, van der Vlugt R, Wetzel T, Yoshikawa N (2020) Proposed revision of the family Secoviridae taxonomy to create three subgenera, “Satsumavirus”, “Stramovirus” and “Cholivirus”, in the genus Sadwavirus. Arch Virol 165:527–533
Sano Y, Wada M, Hashimoto Y, Matsumoto T, Kojima M (1998) Sequences of ten circular ssDNA components associated with the milk vetch dwarf virus genome. J Gen Virol 79:3111–3118
Sasaki M, Shikata E (1977) On some properties of hop stunt disease agent, a viroid. Proc Jpn Acad Ser B 53:109–112
Sasaya T, Ishikawa K, Koganezawa H (2001) Nucleotide sequence of the coat protein gene of Lettuce big-vein virus. J Gen Virol 82:1509–1515
Sasaya T, Kusaba S, Ishikawa K, Koganezawa H (2004) Nucleotide sequence of RNA2 of Lettuce big-vein virus and evidence for a possible transcription termination/initiation strategy similar to that of rhabdoviruses. J Gen Virol 85:2709–2717
Sato K, Yoshikawa N, Takahashi T (1993) Complete nucleotide sequence of the genome of an apple isolate of apple chlorotic leaf spot virus. J Gen Virol 74:1927–1931
Satoh N, Kon T, Yamagishi N, Takahashi T, Natsuaki T, Yoshikawa N (2014) Apple latent spherical virus vector as vaccine for the prevention and treatment of mosaic diseases in pea, broad bean, and eustoma plants by bean yellow mosaic virus. Viruses 6:4242–4257
Saunders K, Bedford ID, Yahara T, Stanley J (2003) Aetiology: the earliest recorded plant virus disease. Nature 422:831
Serra P, Messmer A, Sanderson D, James D, Flores R (2018) Apple hammerhead viroid-like RNA is a bona fide viroid: autonomous replication and structural features support its inclusion as a new member in the genus Pelamoviroid. Virus Res 249:8–15
Shikata E (1974) Rice black-streaked dwarf virus. CMI/AAB descriptions of plant viruses no 135
Shimamoto I, Sonoda S, Vazquez P, Minaka N, Nishiguchi M (1998) Nucleotide sequence analysis of the 3′ terminal region of a wasabi strain of crucifer tobamovirus genomic RNA: Subgrouping of crucifer tobamoviruses. Arch Virol 143:1801–1813
Shimomoto Y, Kobayashi K, Okuda M (2014) Identification and characterization of lisianthus necrotic ringspot virus, a novel distinct tospovirus species causing necrotic disease of lisianthus (Eustoma grandiflorum). J Gen Plant Pathol 80:169–175
Shimomoto Y, Okada T, Ikeda K, Tatara A, Hasegawa Y, Yanagisawa H, Takeyama S, Hayashi K, Yano K, Morita Y, Kubota K (2022) Japanese star anise ringspot-associated virus is a distinct emaravirus transmitted by the eriophyid mite (the family Diptilomiopidae). J Gen Plant Pathol 88:69–80
Shimura H, Masuta C, Yoshida N, Sueda K, Suzuki M (2013) The 2b protein of Asparagus virus 2 functions as an RNA silencing suppressor against systemic silencing to prove functional synteny with related cucumoviruses. Virology 442:180–188
Shirako Y, Suzuki N, French RC (2000) Similarity and divergence among viruses in the genus Furovirus. Virology 270:201–207
Siddell SG, Walker PJ, Lefkowitz EJ, Mushegian AR, Adams MJ, Dutilh BE, Gorbalenya AE, Harrach B, Harrison RL, Junglen S, Knowles NJ, Kropinski AM, Krupovic M, Kuhn JH, Nibert M, Rubino L, Sabanadzovic S, Sanfaçon H, Simmonds P, Varsani A, Zerbini FM, Davison AJ (2019) Additional changes to taxonomy ratified in a special vote by the International Committee on Taxonomy of Viruses (October 2018). Arch Virol 164:943–946
Siddell SG, Walker PJ, Lefkowitz EJ, Mushegian AR, Dutilh BE, Harrach B, Harrison RL, Junglen S, Knowles NJ, Kropinski AM, Krupovic M, Kuhn JH, Nibert ML, Rubino L, Sabanadzovic S, Simmonds P, Varsani A, Zerbini FM, Davison AJ (2020) Binomial nomenclature for virus species: a consultation. Arch Virol 165:519–525
Sumi S, Tsuneyoshi T, Furutani H (1993) Novel rod-shaped viruses isolated from garlic, Allium sativum, possessing a unique genome organization. J Gen Virol 74:1879–1885
Sumi S, Matsumi T, Tsuneyoshi T (1999) Complete nucleotide sequences of garlic viruses A and C, members of the newly ratified genus Allexivirus. Arch Virol 144:1819–1826
Suzuki N (1995) Molecular analysis of the rice dwarf virus genome. Semin Virol 6:89–95
Suzuki S, Hase S, Takahashi H, Ikegami M (2002) The genome organization of pea stem necrosis virus and its assignment to the genus Carmovirus. Intervirology 45:160–163
Takagi M, Goto M, Hisatsune K, Kusano H, Kashima T (2018) Susceptibility of insecticides against melon thrips, Thrips palmi Karny from cucumber greenhouse in Ibaraki Prefecture (in Japanese with English summary). Ann Rep Kanto Tosan Pl Prot Soc 65:115–117
Takai T (1973) Studies on strawberry virus diseases in Japan (in Japanese). Bull Theor Res Stn Ser C (morioka) 8:59–104
Takamura Y, Yamaryo S, Wang WQ, Neriya Y, Suzuki E, Namai K, Ali A, Nishigawa H, Natsuaki T (2020) First report of the complete genomic sequences of strawberry mild yellow edge virus and strawberry vein banding virus isolated in Japan. J Gen Plant Pathol 86:503–506
Takanami Y, Kubo S (1979) Nucleic acids of two phloem-limited viruses: tobacco necrotic dwarf and potato leafroll. J Gen Virol 44:853–856
Takebe I, Otsuki Y (1969) Infection of tobacco mesophyll protoplasts by tobacco mosaic virus. Proc Nat Acad Sci 64:843–848
Takemoto Y, Hibi T (2001) Genes Ia, II, III, IV and V of Soybean chlorotic mottle virus are essential but the gene Ib product is non-essential for systemic infection. J Gen Virol 82:1481–1489
Takemoto Y, Kanehira T, Shinohara M, Yamashita S, Hibi T (2000) The nucleotide sequence and genome organization of Japanese iris necrotic ring virus, a new species in the genus Carmovirus. Arch Virol 145:651–657
Tamada T (2016) General features of beet necrotic yellow vein virus. In: Biancardi E, Tamada T (eds) Rhizomania. Springer, Cham
Tamada T, Kondo H (2013) Biological and genetic diversity of plasmodiophorid-transmitted viruses and their vectors. J Gen Plant Pathol 7:307–320
Tamada T, Goto T, Chiba I, Suwa T (1969) Soybean dwarf, a new virus disease (in Japanese with English summary). Ann Phytopathol Soc Jpn 35:282–285
Tamada T, Uchino H, Kusume T, Iketani-Saito M, Chiba S, Andika IB, Kondo H (2021) Pathogenetic roles of beet necrotic yellow vein virus RNA5 in the exacerbation of symptoms and yield reduction, development of scab-like symptoms, and Rz1-resistance breaking in sugar beet. Plant Pathol 70:219–232
Tan S-H, Nishiguchi M, Murata M, Motoyoshi F (2000) The genome structure of Kyuri green mottle mosaic tobamovirus and its comparison with that of Cucumber green mottle mosaic tobamovirus. Arch Virol 45:1067–1079
Tanina K, Inoue K, Date H, Okuda M, Hanada K, Nasu H, Kasuyama S (2001) Necrotic spot disease of cineraria caused by Impatiens necrotic spot virus (in Japanese with English summary). Jpn J Phytopathol 67:42–45
Tanno F, Nakatsu A, Toriyama S, Kojima M (2000) Complete nucleotide sequence of Northern cereal mosaic virus and its genome organization. Arch Virol 145:1373–1384
Terauchi H, Kanematsu S, Honda K, Mikoshiba Y, Ishiguro K, Hidaka S (2001) Comparison of complete nucleotide sequences of genomic RNAs of four Soybean dwarf virus strains that differ in their vector specificity and symptom production. Arch Virol 146:1885–1898
Tochihara H (1960) Studies on the viruses of Japanese radish mosaic diseases. III. Morphology of cauliflower mosaic virus (in Japanese). Ann Phytopathol Soc Jpn 25:187–192
Tochihara H, Idei T, Yabuki S, Fukumoto F (1975) Properties and distribution of three carnation viruses in Japan (in Japanese with English summary). Ann Phytopathol Soc Jpn 41:390–399
Tomaru M, Maruyama W, Kikuchi A, Yan J, Zhu Y, Suzuki N, Isogai M, Oguma Y, Kimura I, Omura T (1997) The loss of outer capsid protein P2 results in nontransmissibility by the insect vector of rice dwarf phytoreovirus. J Virol 71:8019–8023
Toriyama S (1982) Characterization of rice stripe virus: a heavy component carrying infectivity. J Gen Virol 61:187–195
Toriyama S, Mikoshiba Y, Doi Y (1983) Ryegrass mottle virus, a new virus from Lolium multiflorum in Japan. Ann Phytopathol Soc Jpn 49:610–618
Toriyama S, Kimishima T, Takahashi M (1997) The proteins encoded by rice grassy stunt virus RNA5 and RNA6 are only distantly related to the corresponding proteins of other members of the genus Tenuivirus. J Gen Virol 78:2355–2363
Tsuda S (2021) Plant viruses and viroids infecting crops and its control measures (in Japanese). Shokubutsu Boueki 75:101–112
Ueda S, Kimura T, Onuki M, Hanada K, Iwanami T (2004) Three distinct groups of isolates of Tomato yellow leaf curl virus in Japan and construction of an infectious clone. J Gen Plant Pathol 70:232–238
Uehara-Ichiki T, Nakazono-Nagaoka E, Yamaguchi M, Ohashi M, Kodaira E, Kojima M, Igarashi M, Hanada K, Hishida A, Fujikawa T (2018) Next-generation sequencing and bioassay of viruses in Rehmannia glutinosa. Jpn J Phytopathol 84:151–157 (in Japanese with English abstract)
Uke A, Pinili MS, Natsuaki KT, Geering ADW (2021) Complete genome sequence of aucuba ringspot virus. Arch Virol 166:1227–1230
Uyeda I, Matsumura T, Sano T, Ohshima K, Shikata E (1987) Nucleotide sequence of rice dwarf virus genome segment 10. Proc Jpn Acad Ser B 63:227–230
Uyeda I, Suda N, Yamada N, Kudo H, Murao K, Suga H, Kimura I, Shikata E, Kitagawa Y, Kusano T, Sugawara M, Suzuki N (1994) Nucleotide sequence of rice dwarf phytoreovirus genome segment 2: completion of sequence analyses of rice dwarf virus. Intervirology 37:6–11
Uyeda I, Ando Y, Murao K, Kimura I (1995) High-resolution genome typing and genomic reassortment events of rice dwarf phytoreovirus. Virology 212:724–727
Vaira AM, Accotto GP, Costantini A, Milne RG (2003) The partial sequence of RNA 1 of the ophiovirus Ranunculus white mottle virus indicates its relationship to rhabdoviruses and provides candidate primers for an ophiovirus-specific RT-PCR test. Arch Virol 148:1037–1050
van der Wilk F, Dullemans AM, Verbeek M, van den Heuvel JFJM (2002) Nucleotide sequence and genomic organization of an ophiovirus associated with lettuce big-vein disease. J Gen Virol 83:2869–2877
Van Regenmortel MHV (2019) Solving the species problem in viral taxonomy: recommendations on non-Latinized binomial species names and on abandoning attempts to assign metagenomic viral sequences to species taxa. Arch Virol 164:2223–2229
Wagh SG, Kobayashi K, Yaeno T, Yamaoka N, Masuta C, Nishiguchi M (2016) Rice necrosis mosaic virus, a fungal transmitted Bymovirus: complete nucleotide sequence of the genomic RNAs and subgrouping of bymoviruses. J Gen Plant Pathol 82:38–42
Walker PJ, Siddell SG, Lefkowitz EJ, Mushegian AR, Dempsey DM, Dutilh BE, Harrach B, Harrison RL, Hendrickson RC, Junglen S, Knowles NJ, Kropinski AM, Krupovic M, Kuhn JH, Nibert M, Rubino L, Sabanadzovic S, Simmonds P, Varsani A, Zerbini FM, Davison AJ (2019) Changes to virus taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2019). Arch Virol 164:2417–2429
Wylie SJ, Adams M, Chalam C, Kreuze J, López-Moya JJ, Ohshima K, Praveen S, Rabenstein F, Stenger D, Wang A, Zerbini FM, ICTV Report C (2017) ICTV virus taxonomy profile: Potyviridae. J Gen Virol 98:352–354
Yaegashi H, Oyamada S, Goto S, Yamagishi N, Isogai M, Ito T, Yoshikawa N (2020) Simultaneous infection of sweet cherry with eight virus species including a new foveavirus. J Gen Plant Pathol 86:134–142
Yamamiya A, Miyanishi M, Shirako Y (2005) Stable deletions arising in the readthrough region of Soil-borne wheat mosaic virus RNA2 define the 5′ limit of the functional promoter for the p19 subgenomic RNA. Arch Virol 150:1871–1884
Yamashita S, Doi Y, Yora K, Yoshino M (1979) Cucumber yellows virus: its transmission by the greenhouse whitefly, Trialeurodes vaporariorum (Westwood), and the yellowing disease of cucumber and muskmelon caused by the virus. Ann Phytopathol Soc Jpn 45:484–496
Yamashita S, Natsuaki T, Doi Y, Yora K (1985a) Canna yellow mottle virus, a non-enveloped small bacilliform virus in Canna sp. (in Japanese with English summary). Ann Phytopathol Soc Jpn 51:642–646
Yamashita S, Nonaka N, Namba S, Doi Y, Yora K (1985b) Miscanthus streak virus, a geminivirus in Miscanthus sacchariflorus Benth. et Hook (in Japanese with English summary). Ann Phytopath Soc Jpn 51:582–590
Yamashita K, Sakai J, Hanada K (1996) Characterization of a new virus from garlic (Allium sativum L.), garlic mite-borne mosaic virus. Ann Phytopathol Soc Jpn 62:483–489
Yan J, Tomaru M, Takahashi A, Kimura I, Hibino H, Omura T (1996) P2 protein encoded by genome segment S2 of rice dwarf phytoreovirus is essential for virus infection. Virology 224:539–541
Yan JH, Zhang S, Wu JX, Yang FY, Zhou Y, Zhou CY, Cao MJ (2021) Complete genome sequences and recombination analysis of three divergent satsuma dwarf virus isolates. Trop Plant Pathol 46:26–30
Yanase H (1974) Studies on apple latent viruses in Japan. The association of apple topworking disease with apple latent viruses. Bull Fruit Tree Res Stn Jpn C1:46–109
Yasaka R, Nguyen HD, Ho SYW, Duchêne S, Korkmaz S, Katis N, Takahashi H, Gibbs AJ, Ohshima K (2014) The temporal evolution and global spread of Cauliflower mosaic virus, a plant pararetrovirus. PLoS ONE 9:e85641
Yasaki M, Hirano Y, Uga H, Hanada K, Uehara-Ichiki T, Toda T, Furuya H, Fuji S (2017) Characterization of a new carmovirus isolated from an Adonis plant. Arch Virol 162:501–504
Yonaha T, Toyosato T, Kawano S, Osaki T (1995) Pepper vein yellows virus, a novel luteovirus from bell pepper plants in Japan. Ann Phytopathol Soc Jpn 61:178–184
Yoshida N, Tamada T (2019) Host range and molecular analysis of beet leaf yellowing virus, beet western yellows virus-JP and brassica yellows virus in Japan. Plant Pathol 68:1045–1058
Yoshikawa N, Takahashi T (1988) Properties of RNAs and proteins of apple stem grooving and apple chlorotic leaf spot viruses. J Gen Virol 69:241–245
Yoshikawa N, Sasaki E, Kato M, Takahashi T (1992) The nucleotide sequence of apple stem grooving capillovirus genome. Virology 191:98–105
Yoshikawa N, Imaizumi M, Takahashi T, Inouye N (1993) Striking similarities between the nucleotide sequence and genome organization of citrus tatter leaf and apple stem grooving capilloviruses. J Gen Virol 74:2743–2747
Yoshikawa N, Hibi T, Mayama S, Yamada M, Hyakumachi M, Takahashi K, Ishiguro K, Akimitsu K, Tsushima S (2015) The 100 years history of plant pathology in Japan (in Japanese). Jpn J Phytopathol 81:1–397
Zhang F, Toriyama S, Takahashi M (2001) Complete nucleotide sequence of ryegrass mottle virus: a new species of the genus Sobemovirus. J Gen Plant Pathol 67:63–68
Zhang J, Dey KK, Lin B, Borth WB, Melzer MJ, Sether D, Wang Y, Wang IC, Shen H, Pu X, Sun D, Hu JS (2017) Characterization of Canna yellow mottle virus in a new host, Alpinia purpurata, in Hawaii. Phytopathology 107:791–799
Acknowledgements
The authors are grateful to Dr. Satoshi T. Ohki for providing information on the establishment of the two PSJ committees: the Plant Virus Disease Study Group (PVDSG) and Plant Virus Taxonomy Committee (PVTC). The authors wish to thank the Editor-in-Chief, Dr. Yuki Ichinose for his invitation to contribute this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fuji, Si., Mochizuki, T., Okuda, M. et al. Plant viruses and viroids in Japan. J Gen Plant Pathol 88, 105–127 (2022). https://doi.org/10.1007/s10327-022-01051-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-022-01051-y