Abstract
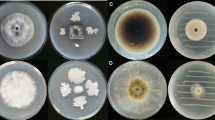
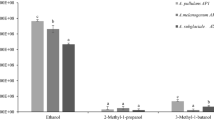
Fruiting bodies of ectomycorrhizal fungi were collected from different areas of Tottori Prefecture, Japan, during 2011–2015, and 18 species were identified by morphological characteristics and molecular analysis. Volatile compounds were extracted with acetone from fruiting bodies, and after concentration, the aqueous residue was extracted with n-hexane. The antifungal activities of the volatile extracts were assayed by inhibition of conidial germination of a phytopathogenic fungus, Alternaria brassicicola; the volatiles from Russula aff. anthracina, R. chloroides and R. senecis completely inhibited conidial at 1 ppm (w/v), the maximum concentration diffused as vapor. In addition, volatile compounds from R. aff. anthracina had remarkable antifungal activity against seven phytopathogenic fungi when tested at vapor concentrations of 0.2 ppm (w/v). The conidia of A. brassicicola did not germinate even after the removal of the volatile compounds, indicating that the active compound had fungicidal activity. The volatile compounds were isolated from the extracts of R. aff. anthracina, and isovelleral was identified as the major antifungal compound. Isovelleral was also detected in the extracts of R. chloroides and R. senecis and had significant antifungal activity against conidial germination of A. brassicicola at a vapor concentration as low as 0.05 ppm (w/v). This is the first report that isovelleral, the volatile compound produced by Russula spp., has antifungal activity against phytopathogenic fungi.




Similar content being viewed by others
References
Aktar MW, Sengupta D, Chowdhury A (2009) Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol 2:1–12
Anke H, Sterner O (1988) Transformation of isovelleral by the parasitic fungus Calcarisporium arbuscula. Phytochemistry 27:2765–2767
Anke H, Sterner O (1991) Comparison of the antimicrobial and cytotoxic activities of twenty unsaturated sesquiterpene dialdehydes from plants and mushrooms. Plant Med 57:344–346
Atmosukarto I, Castillo U, Hess WM, Sears J, Strobel G (2005) Isolation and characterization of Muscodor albus I-41.3s, a volatile antibiotic producing fungus. Plant Sci 169:854–861
Camazine S, Lupo AT Jr (1984) Labile toxic compounds of the lactarii: the role of the laticiferous hyphae as a storage depot for precursors of pungent dialdehydes. Mycologia 76:355–358
Camazine SM, Resch JF, Eisner T, Meinwald J (1983) Mushroom chemical defense: pungent sesquiterpenoid dialdehyde antifeedant to opossum. J Chem Ecol 9:1439–1447
Clericuzio M, Sterner O (1997) Conversion of velutinal esters in the fruit bodies of Russula cuprea. Phytochemistry 45:1569–1572
Fernando PC (2006) Agriculture, pesticides, food security and food safety. Environ Sci Policy 9:685–692
Hansson T, Sterner O, Wickberg B, Bergman R (1992) The thermal isomerization of the sesquiterpenes isovelleral and merulidial. A reversible ring opening of the cis-methylcyclopropanecarboxaldehyde group via an intramolecular ene reaction. J Org Chem 57:3822–3828
Ishii H (2006) Impact of fungicide resistance in plant pathogens on crop disease control and agricultural environment. Jpn Agric Res Q 40:205–211
Koitabashi M, Kajitani Y, Hirashima K (2004) Antifungal substances produced by fungal strain Kyu-W63 from wheat leaf and its taxonomic position. J Gen Plant Pathol 70:124–130
Lee SO, Kim HY, Choi GJ, Lee HB, Jang KS, Choi YH, Kim JG (2009) Mycofumigation with Oxyporus latemarginatus EF069 for control of postharvest apple decay and Rhizoctonia root rot on moth orchid. J Appl Microbiol 106:1213–1219
López-Gutiérrez A, Perez-Moreno J, Hernández-Santiago F, Uscanga-Mortera E, García-Esteva A, Cetina-Alcalá VM, Cardoso-Villanueva MD, Xoconostle-Cázares B (2018) Nutrient mobilization, growth and field survival of Pinus pringlei inoculated with three ectomycorrhizal mushrooms. Bot Sci 96:286–304
Magnusson G, Thoren S, Wickberg B (1972) Fungal extractives I. Structure of a sesquiterpene dialdehyde from Lactarius by computer simulation of the NMR spectrum. Tetrahedron Lett 13:1105–1108
Mercier J, Jiménez JI (2004) Control of fungal decay of apples and peaches by the biofumigant fungus Muscodor albus. Postharvest Biol Technol 31:1–8
Mercier J, Jiménez JI (2007) Potential of the volatile-producing fungus Muscodor albus for control of building molds. Can J Microbiol 53:404–410
Morath SU, Hung R, Bennett JW (2012) Fungal volatile organic compounds: a review with emphasis on their biotechnological potential. Fungal Biol Rev 26:73–83
Nishino S, Parada RY, Ichiyanagi T, Maekawa N, Shimomura N, Otani H (2013) 1-Phenyl-3-pentanone, a volatile compound from the edible mushroom Mycoleptodonoides aitchisonii active against some phytopathogenic fungi. J Phytopathol 161:515–521
Oka K, Shimomura N, Maekawa N, Nakagiri A, Otani H (2014) Antifungal activity of 1-phenyl-3-pentanone produced by Mycoleptodonoides aitchisonii against plant-pathogenic fungi. Mushroom Sci Biotechnol 22:95–100
Oka K, Ishihara A, Sakaguchi N, Nishino S, Parada RY, Nakagiri A, Otani H (2015) Antifungal activity of volatile compounds produced by an edible mushroom Hypsizygus marmoreus against phytopathogenic fungi. J Phytopathol 163:987–996
Petrović J, Glamočlija J, Stojković DS, Ćirić A, Nikolić M, Bukvički D, Guerzoni ME, Soković MD (2013) Laetiporus sulphureus, edible mushroom from Serbia: investigation on volatile compounds, in vitro antimicrobial activity and in situ control of Aspergillus flavus in tomato paste. Food Chem Toxicol 59:297–302
Sterner O, Bergman R, Kihlberg J, Wickberg B (1985) The sesquiterpenes of Lactarius vellereus and their role in a proposed chemical defense system. J Nat Prod 48:279–288
Strobel GA, Dirkse E, Sears J, Markworth C (2001) Volatile antimicrobials from Muscodor albus, a novel endophytic fungus. Microbiology 147:2943–2950
Tomono K, Arimoto Y, Honma Y, Misato Y (1979) Observations of growth stages of Diaporthe citri on epidermal strips of onion bulb. Ann Phytopathol Soc Jpn 45:444–452
Acknowledgements
We thank Dr. H. Ishii of Kibi International University, Dr. Y. Sato of Toyama Prefectural University and Mr. K. Okada of Osaka Prefecture Research Institute of Environment, Agriculture and Fisheries for providing fungal isolates.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Osaki-Oka, K., Suyama, S., Sakuno, E. et al. Antifungal activity of the volatile compound isovelleral produced by ectomycorrhizal Russula fungi against plant-pathogenic fungi. J Gen Plant Pathol 85, 428–435 (2019). https://doi.org/10.1007/s10327-019-00872-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-019-00872-8