Abstract
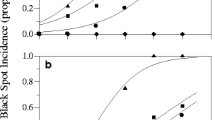
White powdery rot in figs caused by Phytophthora palmivora is an important disease resulting in severe fruit rot, but is not currently effectively controlled in Japan due to a lack of understanding of its epidemiology. Therefore, the effects of temperature, zoospore concentration, infection period, and fruit maturity on infection of figs were examined by inoculating the fruit with a suspension of P. palmivora zoospores. The zoospores germinated at temperatures from 5 to 35 °C, with the optimum temperature range being 20–35 °C. Germ tube length in zoospore cysts was greatest at 20–30 °C. The disease developed in green figs at temperatures from 20 to 30 °C. Figs inoculated with as few as 10 zoospores per fruit developed severe symptoms at the optimum temperature (25 °C). The minimum infection period required for infection was 2 h at 20–28 °C. All of the figs developed symptoms within an 8 h infection period at 25 or 28 °C, and with a 6 h infection period at 25 °C. All fruit at different stages of development (immature fruit, yellow fruit, and mature fruit) developed symptoms. These results indicate that P. palmivora is capable of infecting figs over a wide range of temperatures, within a short infection period, at a low concentration of zoospores, and at any stage of development. These data could be used to construct forecasting models and develop effective control systems for white powdery rot.



Similar content being viewed by others
References
dos Santos AF, Santos F, Mezzomo R, Tessmann DJ, May De Mio LL (2017) First report of fruit rot caused by Phytophthora palmivora on fig in Brazil. Plant Dis 101:1331
EL-Gholl NE, Alfieri SA Jr (1984) Fruit rot of fig caused by Phytophthora palmivora. Proc Fla State Hort Soc 97:327–328
Elmer WH (2002) Influence of inoculum density of Fusarium oxysporum f. sp. cyclaminis and sodium chloride on cyclamen and the development of Fusarium wilt. Plant Dis 86:389–393
Erwin DC, Ribeiro OK (eds) (1996) Phytophthora diseases worldwide. APS Press, St. Paul, MN, USA
Fall ML, Tremblay DM, Gobeil-Richard M, Couillard J, Rocheleau H, Van der Heyden H, Lévesque CA, Beaulieu C, Carisse O (2015) Infection efficiency of four Phytophthora infestans clonal lineages and DNA-based quantification of sporangia. PLoS One 10:e0136312
Gappa-Adachi R, Yano K, Takeuchi S, Morita Y, Uematsu S (2012) Phytophthora blight of southern star (Oxypetalum caeruleum) caused by Phytophthora palmivora in Japan. J Gen Plant Pathol 78:39–42
Gongora-Canul CC, Leandro LFS (2011a) Plant age affects root infection and development of foliar symptoms of soybean sudden death syndrome. Plant Dis 95:242–247
Gongora-Canul CC, Leandro LFS (2011b) Effect of soil temperature and plant age at time of inoculation on progress of root rot and foliar symptoms of soybean sudden death syndrome. Plant Dis 95:436–440
Grove GG, Madden LV, Ellis MA, Schmitthenner AF (1985) Influence of temperature and wetness duration on infection of immature strawberry fruit by Phytophthora cactorum. Phytopathology 75:165–169
Hori S (1915) Phytophthora fici. J Plant Prot 2:930–932 (in Japanese)
Hunter JE, Kunimoto RK (1974) Dispersal of Phytophthora palmivora sporangia by wind-blown rain. Phytopathology 64: 202–206
Ko WH, Chan MJ (1974) Infection and colonization potential of sporangia, zoospores, and chlamydospores of Phytophthora palmivora in soil. Phytopathology 64:1307–1309
Ko WH, Chase LL, Kunimoto RK (1973) A microsyringe method for determining concentration of fungal propagules. Phytopathology 63:1206–1207
Minogue KP, Fry WE (1981) Effect of temperature, relative humidity, and rehydration rate on germination of dried sporangia of Phytophthora infestans. Phytopathology 71:1181–1184
Miyake N, Ito S (2005) Seasonal occurrence of fruit rots of fig fields. Ann Rept Kansai Pl Prot 47:87–89 (in Japanese)
Miyake N, Nagai H (2017) Efficacy of phosphonate in controlling white powdery rot of fig caused by Phytophthora palmivora. J Gen Plant Pathol 83:390–397
Qu T, Shao Y, Csinos AS, Ji P (2016) Sensitivity of Phytophthora nicotianae from tobacco to fluopicolide, mandipropamid, and oxathiapiprolin. Plant Dis 100:2119–2125
Rao VG (1970) Influence of temperature upon growth and sporulation in two species of Phytophthora. Mycopathol Mycol Appl 42: 39–48
Riedel M, Werres S, Elliott M, McKeever K, Shamoun SF (2012) Histopathological investigations of the infection process and propagule development of Phytophthora ramorum on rhododendron leaves. For Phytophthoras 2: https://doi.org/10.5399/osu/fp.2.1.3036
Sarria GA, Martinez G, Varon F, Drenth A, Guest DI (2016) Histopathological studies of the process of Phytophthora palmivora infection in oil palm. Eur J Plant Pathol 145:39–51
Sato N (1994) Effect of water temperature on direct germination of the sporangia of Phytophthora infestans. Ann Phytopath Soc Japan 60:162–166
Sparks AH, Esker PD, Bates M, Dall’ Acqua W, Guo Z, Segovia V, Silwal SD, Tolos S, Garrett KA (2008) Calculating the area under the disease progress curve to quantify disease progress. In: Disease Progress over Time. The American Phytopathological Society. http://www.apsnet.org/EDCENTER/ADVANCED/TOPICS/ECOLOGYANDEPIDEMIOLOGYINR/DISEASEPROGRESS/Pages/AUDPC.aspx. Accessed 3 July 2017
Timmer LW, Zitko SE, Gottwald TR, Graham JH (2000) Phytophthora brown rot of citrus: temperature and moisture effects on infection, sporangium production, and dispersal. Plant Dis 84:157–163
Widmer TL (2009) Infective potential of sporangia and zoospores of Phytophthora ramorum. Plant Dis 93:30–35
Zhang C, Zhang W, Ma HQ, Zhang GZ (2013) First report of Phytophthora palmivora causing fruit rot of fig (Ficus carica L.) in China. Plant Dis 97:1252
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Miyake, N., Nagai, H. Effects of temperature, zoospore concentration, infection period, and fruit maturity on Phytophthora palmivora infection of figs. J Gen Plant Pathol 84, 330–338 (2018). https://doi.org/10.1007/s10327-018-0796-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-018-0796-1