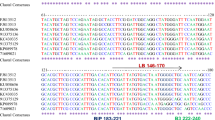
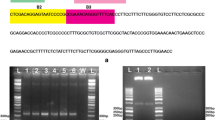
Abstract
An accurate and reliable system to detect Potato spindle tuber viroid (PSTVd) was developed based on duplex reverse transcription-polymerase chain reaction (RT-PCR) in combination with a plant mRNA internal control to evaluate the quality of RNA extracted from a dormant potato tuber and the effectiveness of the RT-PCR. In addition, a non-infectious RNA based on the PSTVd sequence was generated in vitro as a readily available positive control without the possibility of escape; this RNA was distinguishable from true positives in PSTVd infection. This detection system will be useful in plant quarantine and phytosanitary certification applications.




Similar content being viewed by others
References
Di Serio F (2007) Identification and characterization of Potato spindle tuber viroid infecting Solanum jasminoides and S. rantonnetii in Italy. J Plant Pathol 89:297–300
Elliott DR, Alexander BJR, Smales TE, Tang Z, Clover GRG (2001) First report of Potato spindle tuber viroid in tomato in New Zealand. Plant Dis 85:1027
Gross HJ, Domdey H, Lossow C, Jank P, Raba M, Alberty H, Sänger HL (1978) Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature 273:203–208
Hammond RW, Owens RA (2006) Viroids: new and continuing risks for horticultural and agricultural crops. APSnet Features November 2006 [http://apsnet.org/online/feature/viroids/], American Phytopathological Society, St. Paul, Minnesota
Hataya T, Inoue AK, Shikata E (1994) A PCR-microplate hybridization method for plant virus detection. J Virol Methods 46:223–236
Lebas BSM, Clover GRG, Ochoa-Corona FM, Elliott DR, Tang Z, Alexander BJR (2005) Distribution of Potato spindle tuber viroid in New Zealand glasshouse crops of capsicum and tomato. Australas Plant Pathol 34:129–133
Maoka T, Hataya T (2005) Simultaneous detection of twelve potato viruses using a cDNA macroarray (abstract in Japanese). Jpn J Phytopathol 71:236
Matsushita Y, Kanda A, Usugi T, Tsuda S (2008) First report of a Tomato chlorotic dwarf viroid disease on tomato plants in Japan. J Gen Plant Pathol 74:182–184
Mumford RA, Jarvis B, Skelton A (2004) The first report of Potato spindle tuber viroid (PSTVd) in commercial tomatoes in the UK. Plant Pathol 53:242
Nakahara K, Hataya T, Kimura I, Shikata E (1997) Reactions of potato cultivars in Japan to potato spindle tuber viroid and its gene diagnosis (in Japanese with English summary). Annu Rep Soc Plant Prot North Jpn 48:69–74
Nakahara K, Hataya T, Hayashi Y, Sugimoto T, Kimura I, Shikata E (1998) A mixture of synthetic oligonucleotide probes labeled with biotin for the sensitive detection of potato spindle tuber viroid. J Virol Methods 71:219–227
Nakahara K, Hataya T, Uyeda I (1999) A simple, rapid method of nucleic acid extraction without tissue homogenization for detecting viroids by hybridization and RT-PCR. J Virol Methods 77:47–58
Nassuth A, Pollari E, Helmeczy K, Stewart S, Kofalvi SA (2000) Improved RNA extraction and one-tube RT-PCR assay for simultaneous detection of control plant RNA plus several viruses in plant extracts. J Virol Methods 90:37–49
Owens RA (2007) Potato spindle tuber viroid: the simplicity paradox resolved? Mol Plant Pathol 8:549–560
Puchta H, Herold T, Verhoeven K, Roenhorst A, Ramm K, Schmidt-Puchta W, Sänger HL (1990) A new strain of potato spindle tuber viroid (PSTVd-N) exhibits major sequence differences as compared to all other PSTVd strains sequenced so far. Plant Mol Biol 15:509–511
Querci M, Owens RA, Vargas C, Salazar LF (1995) Detection of potato spindle tuber viroid in avocado growing in Peru. Plant Dis 79:196–202
Salazar LF, Bartolini L, Hurtado A (2001) Viroids. In: Loebenstein G, Berger PH, Brunt AA, Lawson RH (eds) Virus and virus-like diseases of potatoes and production of seed-potatoes. Kluwer, Dordrecht, pp 135–144
Sato M, Hataya T, Iwasaki M (2000) Detection of four viruses in dormant potato tubers by RT-PCR (in Japanese with English summary). Annu Rep Soc Plant Prot North Jpn 51:87–92
Shamloul AM, Hadidi A, Zhu SF, Singh RP, Sagredo B (1997) Sensitive detection of potato spindle tuber viroid using RT-PCR and identification of a viroid variant naturally infecting pepino plants. Can J Plant Pathol 19:89–96
Thompson JR, Wetzel S, Klerks MM, Vašková D, Schoen CD, Špak J, Jelkmann W (2003) Multiplex RT-PCR detection of four aphid-borne strawberry viruses in Fragaria spp. in combination with a plant mRNA specific internal control. J Virol Methods 111:85–93
Verhoeven JThJ, Jansen CCC, Roenhorst JW (2007a) First report of pospiviroids infecting ornamentals in the Netherlands: Citrus exocortis viroid in Verbena sp., Potato spindle tuber viroid in Brugmansia suaveolens and Solanum jasminoides, and Tomato apical stunt viroid in Cestrum sp. New Disease Reports 15 [http://www.bspp.org.uk/ndr/july2007/2007-13.asp]
Verhoeven JThJ, Jansen CCC, Roenhorst JW (2007b) Streptosolen jamesonii ‘Yellow’, a new host plant of Potato spindle tuber viroid. New Disease Reports 15 [http://www.bspp.org.uk/ndr/july2007/2007-46.asp]
Verhoeven JThJ, Jansen CCC, Roenhorst JW, Steyer S, Michelante D (2007c) First report of Potato spindle tuber viroid in tomato in Belgium. Plant Dis 91:1055
Verhoeven JThJ, Jansen CCC, Willemen TM, Kox LFF, Owens RA, Roenhorst JW (2004) Natural infections of tomato by Citrus exocortis viroid, Columnea latent viroid, Potato spindle tuber viroid and Tomato chlorotic dwarf viroid. Eur J Plant Pathol 110:823–831
Wassenegger M, Heimes S, Sänger HL (1994) An infectious viroid RNA replicon evolved from an in vitro-generated non-infectious viroid deletion mutant via a complementary deletion in vivo. EMBO J 13:6172–6177
Acknowledgments
This work was supported in part by a grant-in-aid from the Agriculture, Forestry, and Fisheries Research Council, MAFF of Japan (No. 1722).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hataya, T. Duplex reverse transcription-polymerase chain reaction system to detect Potato spindle tuber viroid using an internal control mRNA and a non-infectious positive control RNA. J Gen Plant Pathol 75, 167–172 (2009). https://doi.org/10.1007/s10327-009-0159-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-009-0159-z