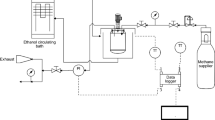
Abstract
The formation of gas hydrates is a major issue during the operation of oil and gas pipelines, because gas hydrates cause plugging, thereby disrupting the normal oil and gas flows. A solution is to inject gas hydrate inhibitors such as ionic liquids. Contrary to classical inhibitors, ionic liquids act both as thermodynamic inhibitors and hydrate inhibitors, and as anti-agglomerates. Imidazolium-based ionic liquids have been found efficient for the inhibition of CO2 and CH4 hydrates. For CO2 gas hydrates, N-ethyl-N-methylmorpholinium bromide showed an average depression temperature of 1.72 K at 10 wt% concentration. The induction time of 1-ethyl-3-methyl imidazolium bromide is 36.3 h for CO2 hydrates at 1 wt% concentration. For CH4 hydrates, 1-ethyl-3-methyl-imidazolium chloride showed average depression temperature of 4.80 K at 40 wt%. For mixed gas hydrates of CO2 and CH4, only quaternary ammonium salts have been studied. Tetramethyl ammonium hydroxide shifted the hydrate liquid vapour equilibrium to 1.56 K at 10 wt%, while tetrabutylammonium hydroxide showed an induction time of 0.74 h at 1 wt% concentration.







Similar content being viewed by others
Abbreviations
- AILs:
-
Ammonium-based ionic liquids
- DSC:
-
Differential scanning calorimetry
- GHI:
-
Gas hydrate inhibitor
- ILs:
-
Ionic liquids
- KHI:
-
Kinetic hydrate inhibitor
- LDHIs:
-
Low-dose hydrate inhibitors
- MEG:
-
Monoethylene glycol
- mf:
-
Mass fraction
- PVCap:
-
Polyvinyl caprolactam
- PVP:
-
Polyvinylpyrrolidone
- QAS:
-
Quaternary ammonium salts
- RIP:
-
Relative inhibition power
- SNG:
-
Standard natural gas
- sI, sII, sH:
-
Structure I, II, H
- THI:
-
Thermodynamic hydrate inhibitor
References
Ahmed I (2021) Dual-functional gas hydrate inhibition of tetramethylammonium chloride for carbon dioxide-methane mixed gas systems. Fuel 305:121598. https://doi.org/10.1016/j.fuel.2021.121598
Altamash T, Aparicio S, Atilhan M (2019) An experimental study on doubly salt effect for methane hydrate inhibition. J Nat Gas Sci Eng 72:103015. https://doi.org/10.1016/j.jngse.2019.103015
Bai Y, Bai Q (2018) Subsea engineering handbook, 2nd edn. Gulf Professional Publishing, Houston
Bavoh CB, Lal B, Keong LK et al (2016a) Synergic kinetic inhibition effect of EMIM-Cl + PVP on CO2 hydrate formation. Procedia Eng 148:1232–1238. https://doi.org/10.1016/j.proeng.2016.06.474
Bavoh CB, Lal B, Nashed O et al (2016b) COSMO-RS: An ionic liquid prescreening tool for gas hydrate mitigation. Chin J Chem Eng 24:1619–1624. https://doi.org/10.1016/j.cjche.2016.07.014
Bavoh CB, Partoon B, Lal B, Kok Keong L (2017) Methane hydrate-liquid–vapour-equilibrium phase condition measurements in the presence of natural amino acids. J Nat Gas Sci Eng 37:425–434. https://doi.org/10.1016/j.jngse.2016.11.061
Bavoh CB, Lal B, Ben-Awuah J et al (2019) Kinetics of mixed amino acid and ionic liquid on CO2 hydrate formation. IOP Conf Ser Mater Sci Eng 495:1–8. https://doi.org/10.1088/1757-899X/495/1/012073
Bozorgian A (2021) An overview of methane gas hydrate formation. J Eng Ind Res 2:166–177
Cha M, Shin K, Kim J et al (2013) Thermodynamic and kinetic hydrate inhibition performance of aqueous ethylene glycol solutions for natural gas. Chem Eng Sci 99:184–190. https://doi.org/10.1016/j.ces.2013.05.060
Cha JH, Ha C, Kang SP et al (2016) Thermodynamic inhibition of CO2 hydrate in the presence of morpholinium and piperidinium ionic liquids. Fluid Phase Equilib 413:75–79. https://doi.org/10.1016/j.fluid.2015.09.008
Chen W (2011) Status and challenges of Chinese deepwater oil and gas development. Pet Sci 8:477–484. https://doi.org/10.1007/s12182-011-0171-8
Chen Q, Yu Y, Zeng P et al (2008) Effect of 1-butyl-3-methylimidazolium tetrafluoroborate on the formation rate of CO2 hydrate. J Nat Gas Chem 17:264–267. https://doi.org/10.1016/S1003-9953(08)60061-4
Chong ZR, Yang SHB, Babu P et al (2016) Review of natural gas hydrates as an energy resource: prospects and challenges. Appl Energy 162:1633–1652. https://doi.org/10.1016/j.apenergy.2014.12.061
Chu CK, Lin ST, Chen YP et al (2016) Chain length effect of ionic liquid 1-alkyl-3-methylimidazolium chloride on the phase equilibrium of methane hydrate. Fluid Phase Equilib 413:57–64. https://doi.org/10.1016/j.fluid.2015.10.007
Chun LK, Jaafar A (2013) Ionic liquid as low dosage hydrate inhibitor for flow assurance in pipeline. Asian J Sci Res 6:374–380. https://doi.org/10.3923/ajsr.2013.374.380
Daraboina N, Malmos C, Von Solms N (2013a) Synergistic kinetic inhibition of natural gas hydrate formation. Fuel 108:749–757. https://doi.org/10.1016/j.fuel.2013.02.018
Daraboina N, Malmos C, Von Solms N (2013b) Investigation of kinetic hydrate inhibition using a high pressure micro differential scanning calorimeter. Energy Fuels 27:5779–5786. https://doi.org/10.1021/ef401042h
Dashti H, Zhehao Yew L, Lou X (2015) Recent advances in gas hydrate-based CO2 capture. J Nat Gas Sci Eng 23:195–207. https://doi.org/10.1016/j.jngse.2015.01.033
de Menezes DÉS, Pessôa Filho PA, Robustillo Fuentes MD (2020) Use of 1-butyl-3-methylimidazolium-based ionic liquids as methane hydrate inhibitors at high pressure conditions. Chem Eng Sci. https://doi.org/10.1016/j.ces.2019.115323
Drive C, Carroll J (2009) "Natural gas hydrates: " a guide for engineers, 3rd edn. Elsevier, Amsterdam
Farhadian A, Varfolomeev MA, Rahimi A et al (2021) Gas hydrate and corrosion inhibition performance of the newly synthesized polyurethanes: potential dual function inhibitors. Energy Fuels 35:6113–6124. https://doi.org/10.1021/acs.energyfuels.1c00101
Foo KS, Khan MS, Lal B, Sufian S (2018) Semi-clathratic impact of tetrabutylammonium hydroxide on the carbon dioxide hydrates. IOP Conf Ser Mater Sci Eng. https://doi.org/10.1088/1757-899X/458/1/012060
Fournaison L, Delahaye A, Chatti I (2004) General research of CO2 hydrates in Refrigeration processes. Carbon 43:6521–6526
Gambelli AM, Tinivella U, Giovannetti R et al (2021) Observation of the main natural parameters influencing the formation of gas hydrates. Energies 14:1–25. https://doi.org/10.3390/en14071803
Golombok M, Ineke E (2009) Resolving CO2 and methane hydrate formation kinetics. Environ Chem Lett 7:325–330. https://doi.org/10.1007/s10311-008-0173-y
Harrison SE (2010) Natural gas hydrates. InTech, Rijeka
Hassanpouryouzband A, Joonaki E, Vasheghani Farahani M et al (2020) Gas hydrates in sustainable chemistry. Chem Soc Rev 49:5225–5309. https://doi.org/10.1039/c8cs00989a
Huo Z, Freer E, Lamar M et al (2001) Hydrate plug prevention by anti-agglomeration. Chem Eng Sci 56:4979–4991. https://doi.org/10.1016/S0009-2509(01)00188-9
Hussain HH, Husin H (2020) Review on application of quaternary ammonium salts for gas hydrate inhibition. Appl Sci (switzerland) 10:1–12. https://doi.org/10.3390/app10031011
Hussain H, Husin H (2021) The effect of synergistic amino acids-ionic liquids in methane hydrate inhibition by COSMO-RS application. J Mol Liq. https://doi.org/10.1016/j.molliq.2020.114837
Jensen L, Thomsen K, Von Solms N (2011) Inhibition of structure I and II gas hydrates using synthetic and biological kinetic inhibitors. Energy Fuels 25:17–23. https://doi.org/10.1021/ef100833n
Kannan K, Punase A (2009) Low dosage, high efficiency and environment friendly inhibitors: a new horizon in gas hydrates mitigation in production systems. In: Society of petroleum engineers—EUROPEC/EAGE conference and exhibition
Ke W, Chen D (2019) A short review on natural gas hydrate, kinetic hydrate inhibitors and inhibitor synergists. Chin J Chem Eng 27:2049–2061. https://doi.org/10.1016/j.cjche.2018.10.010
Ke W, Kelland MA (2016) Kinetic hydrate inhibitor studies for gas hydrate systems: a review of experimental equipment and test methods. Energy Fuels 30:10015–10028. https://doi.org/10.1021/acs.energyfuels.6b02739
Kelland MA (2006) History of the development of low dosage hydrate inhibitors. Energy Fuels 20:825–847. https://doi.org/10.1021/ef050427x
Kelland MA (2014) Production chemicals for the oil and gas industry, 2nd edn. CRC Press, New York
Keshavarz L, Javanmardi J, Eslamimanesh A, Mohammadi AH (2013) Experimental measurement and thermodynamic modeling of methane hydrate dissociation conditions in the presence of aqueous solution of ionic liquid. Fluid Phase Equilib 354:312–318. https://doi.org/10.1016/j.fluid.2013.05.007
Khan MS, Lal B, Partoon B et al (2016) Experimental evaluation of a novel thermodynamic inhibitor for CH4 and CO2 hydrates. Procedia Eng 148:932–940. https://doi.org/10.1016/j.proeng.2016.06.433
Khan MS, Bavoh CB, Partoon B et al (2017a) Thermodynamic effect of ammonium based ionic liquids on CO2 hydrates phase boundary. J Mol Liq 238:533–539. https://doi.org/10.1016/j.molliq.2017.05.045
Khan MS, Lal B, Bavoh CB et al (2017b) Influence of ammonium based compounds for gas hydrate mitigation: a short review. Indian J Sci Technol 10:1–6. https://doi.org/10.17485/ijst/2017/v10i5/99734
Khan MS, Partoon B, Bavoh CB et al (2017c) Influence of tetramethylammonium hydroxide on methane and carbon dioxide gas hydrate phase equilibrium conditions. Fluid Phase Equilib 440:1–8. https://doi.org/10.1016/j.fluid.2017.02.011
Khan MS, Bavoh CB, Partoon B et al (2018a) Impacts of ammonium based ionic liquids alkyl chain on thermodynamic hydrate inhibition for carbon dioxide rich binary gas. J Mol Liq 261:283–290. https://doi.org/10.1016/j.molliq.2018.04.015
Khan MS, Cornelius BB, Lal B, Bustam MA (2018b) Kinetic assessment of tetramethyl ammonium hydroxide (ionic liquid) for carbon dioxide. Methane Binary Mix Gas Hydrates. https://doi.org/10.5772/intechopen.77262
Khan MS, Lal B, Keong LK, Sabil KM (2018c) Experimental evaluation and thermodynamic modelling of AILs alkyl chain elongation on methane riched gas hydrate system. Fluid Phase Equilib 473:300–309. https://doi.org/10.1016/j.fluid.2018.07.003
Khan MS, Yaqub S, Manner N et al (2018d) Experimental equipment validation for methane (CH4) and carbon dioxide (CO2) hydrates. IOP Conf Ser Mater Sci Eng. https://doi.org/10.1088/1757-899X/344/1/012025
Khan MS, Lal B, Keong LK, Ahmed I (2019a) Tetramethyl ammonium chloride as dual functional inhibitor for methane and carbon dioxide hydrates. Fuel 236:251–263. https://doi.org/10.1016/j.fuel.2018.09.001
Khan MS, Lal B, Shariff AM, Mukhtar H (2019b) Ammonium hydroxide ILs as dual-functional gas hydrate inhibitors for binary mixed gas (carbon dioxide and methane) hydrates. J Mol Liq 274:33–44. https://doi.org/10.1016/j.molliq.2018.10.076
Kim NJ, Park SS, Kim HT, Chun W (2011a) A comparative study on the enhanced formation of methane hydrate using CM-95 and CM-100 MWCNTs. Int Commun Heat Mass Transf 38:31–36. https://doi.org/10.1016/j.icheatmasstransfer.2010.10.002
Kim SM, Lee JD, Lee HJ et al (2011b) Gas hydrate formation method to capture the carbon dioxide for pre-combustion process in IGCC plant. Int J Hydrog Energy 36:1115–1121. https://doi.org/10.1016/j.ijhydene.2010.09.062
Kiran BS, Prasad PSR (2021) Inhibition of methane hydrates using biodegradable additives. ACS Omega 6:8261–8270. https://doi.org/10.1021/acsomega.0c06328
Koh CA, Savidge JL, Tang CC (1996) Time-resolved in-situ experiments on the crystallization of natural gas hydrates. J Phys Chem 100:6412–6414. https://doi.org/10.1021/jp960094s
Koh CA, Westacott RE, Zhang W et al (2002) Mechanisms of gas hydrate formation and inhibition. Fluid Phase Equilib 194–197:143–151. https://doi.org/10.1016/S0378-3812(01)00660-4
Koh CA, Sloan E, Sum A (2011a) Natural gas hydrates in flow assurance. Nat Gas Hydrates in Flow Assur. https://doi.org/10.1016/C2009-0-62311-4
Koh CA, Sloan ED, Sum AK, Wu DT (2011b) Fundamentals and applications of gas hydrates. Annu Re Chem Biomol Eng 2:237–257. https://doi.org/10.1146/annurev-chembioeng-061010-114152
Koh CA, Sum AK, Sloan ED (2012) State of the art: natural gas hydrates as a natural resource. J Nat Gas Sci Eng 8:132–138. https://doi.org/10.1016/j.jngse.2012.01.005
Krishnan A, Panchamoorthy K, Dai G et al (2020) Ionic liquids, deep eutectic solvents and liquid polymers as green solvents in carbon capture technologies: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-020-01057-y
Kurnia KA, Quental MV, Santos LMNBF et al (2015) Mutual solubilities between water and non-aromatic sulfonium ammonium- and phosphonium-hydrophobic ionic liquids. Phys Chem Chem Phys 17:4569–4577. https://doi.org/10.1039/c4cp05339g
Kvamme B (2012) Symposium on natural gas hydrates: a future climate bomb or a valuable source of energy opportunities and challenges for the computational physics community. AIP Conf Proc 1504:754. https://doi.org/10.1063/1.4771804
Lal B, Nashed O (2020) Chemical additives for gas hydrates, 1st edn. Springer, Berlin
Lal B, Qasim A, Sharif AM (2021) Ionic liquids in flow assurance, 1st edn. Springer, Cham
Li S, Li Y, Yang L et al (2021) Prediction of equilibrium conditions for gas hydrates in the organic inhibitor aqueous solutions using a thermodynamic consistency-based model. Fluid Phase Equilib 544–545:113118. https://doi.org/10.1016/j.fluid.2021.113118
Lim VWS, Metaxas PJ, Johns ML et al (2021) The delay of gas hydrate formation by kinetic inhibitors. Chem Eng J 411:128478. https://doi.org/10.1016/j.cej.2021.128478
Lin Y, Rui H, Zhang L, Fang D et al (2018) Quaternary phosphonium cationic ionic liquid/porous metal–organic framework as an efficient catalytic system for cycloaddition of carbon dioxide into cyclic carbonates. Environ Chem Lett. https://doi.org/10.1007/s10311-018-0793-9
Liu Y, Zhao Y, Jia Y et al (2021) Understanding the inhibition performance of kinetic hydrate inhibitors in nanoclay systems. Chem Eng J 424:130303. https://doi.org/10.1016/j.cej.2021.130303
Long Z, He Y, Zhou X et al (2017) Phase behavior of methane hydrate in the presence of imidazolium ionic liquids and their mixtures. Fluid Phase Equilib 439:1–8. https://doi.org/10.1016/j.fluid.2017.02.008
Makino T, Matsumoto Y, Sugahara T et al (2011) Effect of ionic liquid on hydrate formation rate in research center for compact chemical system national institute of advanced industrial science and technology yuuki Matsumoto. Takeshi Sugahara, Kazunariohgaki Osaka University Department of Applied ch. 2–5
May EF, Wu R, Kelland MA et al (2014) Quantitative kinetic inhibitor comparisons and memory effect measurements from hydrate formation probability distributions. Chem Eng Sci 107:1–12. https://doi.org/10.1016/j.ces.2013.11.048
Mohamed NA, Tariq M, Atilhan M et al (2017) Investigation of the performance of biocompatible gas hydrate inhibitors via combined experimental and DFT methods. J Chem Thermodyn 111:7–19. https://doi.org/10.1016/j.jct.2017.03.013
Moradi MR, Razavi K, Company G et al (2011) A thermodynamic study of methane hydrate formation in the presence of (Bmim)(BF4) and (Bmim)(MS) ionic liqiuids
Nallakukkala S, Lal B (2021) Seawater and produced water treatment via gas hydrate: review. J Environ Chem Eng 9:105053. https://doi.org/10.1016/j.jece.2021.105053
Nallakukkala S, Kassim Z, Othman NA, Lal B (2021) Advancement in gas hydrate water based produced water desalination: an overview. 200:190–197. https://doi.org/10.2991/aer.k.201229.027
Nashed O, Sabil KM, Lal B et al (2014) Study of 1-(2-hydroxyethyle) 3-methylimidazolium halide as thermodynamic inhibitors. Appl Mech Mater 625:337–340
Nashed O, Sabil KM, Ismail L et al (2018) Mean induction time and isothermal kinetic analysis of methane hydrate formation in water and imidazolium based ionic liquid solutions. J Chem Thermodyn 117:147–154. https://doi.org/10.1016/j.jct.2017.09.015
Nazari K, Ahmadi AN (2011) A thermodynamic study of methane hydrate formation in the presence of (Bmim)(BF4 and (Bmim)(Ms) ionic liquids. In: I7th Intenational confrence on gas hydrates (ICGH) 2011 9
Nesterov AN, Reshetnikov AM, Manakov AY et al (2015) Promotion and inhibition of gas hydrate formation by oxide powders. J Mol Liq 204:118–125. https://doi.org/10.1016/j.molliq.2015.01.037
Obanijesu EO (2012) Corrosion and hydrate formation in natural gas pipelines. Curtin University, Bentley
Obanijesu EO, Pareek V, Tade MO (2010) Hydrate formation and its influence on natural gas pipeline internal corrosion rate. Environment 62:164–173. https://doi.org/10.2118/128544-MS
Ohno H, Moudrakovski I, Gordienko R et al (2012) Structures of hydrocarbon hydrates during formation with and without inhibitors. J Phys Chem A 116:1337–1343. https://doi.org/10.1021/jp210714m
Partoon B, Wong NMS, Sabil KM et al (2013) A study on thermodynamics effect of (EMIM)-Cl and (OH-C2MIM)-Cl on methane hydrate equilibrium line. Fluid Phase Equilib 337:26–31. https://doi.org/10.1016/j.fluid.2012.09.025
Qasim A, Khan MS, Lal B, Shariff AM (2019a) A perspective on dual purpose gas hydrate and corrosion inhibitors for flow assurance. J Pet Sci Eng 183:106418. https://doi.org/10.1016/j.petrol.2019.106418
Qasim A, Khan MS, Lal B, Shariff AM (2019b) Phase equilibrium measurement and modeling approach to quaternary ammonium salts with and without monoethylene glycol for carbon dioxide hydrates. J Mol Liq 282:106–114. https://doi.org/10.1016/j.molliq.2019.02.115
Qasim A, Khan MS, Lal B et al (2020) Quaternary ammonium salts as thermodynamic hydrate inhibitors in the presence and absence of monoethylene glycol for methane hydrates. Fuel 259:116219. https://doi.org/10.1016/j.fuel.2019.116219
Qureshi MF, Atilhan M, Altamash T et al (2016) Gas hydrate prevention and flow assurance by using mixtures of ionic liquids and synergent compounds: combined kinetics and thermodynamic approach. Energy Fuels 30:3541–3548. https://doi.org/10.1021/acs.energyfuels.5b03001
Rajadurai V, Lakshmi B (2020) Ionic liquids to remove toxic metal pollution. Environ Chem Lett. https://doi.org/10.1007/s10311-020-01115-5
Rajput F, Maric M, Servio P (2021) Amphiphilic block copolymers with vinyl caprolactam as kinetic gas hydrate inhibitors. Energies. https://doi.org/10.3390/en14co020341
Ratti R (2014) Ionic liquids: synthesis and applications in catalysis. Adv Chem 2014:1–16. https://doi.org/10.1155/2014/729842
Rehman A, Zaini DB, Lal B (2021) Application of gas hydrate based technique in wastewater treatment—a mini review. 200:249–254. https://doi.org/10.2991/aer.k.201229.033
Richard AR, Adidharma H (2013) The performance of ionic liquids and their mixtures in inhibiting methane hydrate formation. Chem Eng Sci 87:270–276. https://doi.org/10.1016/j.ces.2012.10.021
Sa JH, Sum AK (2021) Universal correlation for gas hydrates suppression temperature of inhibited systems: IV. Water activity. AIChE J. https://doi.org/10.1002/aic.17293
Sabil KM, Nashed O, Lal B et al (2015) Experimental investigation on the dissociation conditions of methane hydrate in the presence of imidazolium-based ionic liquids. J Chem Thermodyn 84:7–13. https://doi.org/10.1016/j.jct.2014.12.017
Sahith SJK, Pedapati SR, Lal B (2020a) Investigation on gas hydrates formation and dissociation in multiphase gas dominant transmission pipelines. Appl Sci. https://doi.org/10.3390/app10155052
Sahith SJK, Yadavalli SS, Mamidi LT, Kamireddi VR (2020b) Investigation on the kinetic behavior of gas hydrates based on induction time for a high CO2 mixed gas multiphase pipeline system. In: Society of petroleum engineers—Abu Dhabi international petroleum exhibition and conference 2020, ADIP 2020. https://doi.org/10.2118/203121-ms
Sahith SJK, Sivabalan V, Pedapati SR, Lal B (2021) Investigation of CO2 hydrate formation in the presence of gasoline. 200:125–131. https://doi.org/10.2991/aer.k.201229.018
Sahweity MAS (2020) Hydrate management controls in Saudi Aramco’s Largest offshorenonassociated gas fields. In: Proceedings of the annual offshore technology conference 2020-May. https://doi.org/10.4043/30768-ms
Sayani JKS, Pedapati SR, Kassim Z, Lal B (2021) Investigation on thermodynamic equilibrium conditions of methane hydrates in multiphase gas-dominant pipelines. ACS Omega 6:2505–2512. https://doi.org/10.1021/acsomega.0c04204
Sayed MA, Saini RK, AlAli E et al (2021) Safer dual-functional gas hydrate dissolver and inhibitor to replace methanol. Energy Fuels. https://doi.org/10.1021/acs.energyfuels.1c01781
Shen XD, Long Z, Shi LL, Liang DQ (2015) Phase equilibria of CO2 hydrate in the aqueous solutions of N-butyl-N-methylpyrrolidinium bromide. J Chem Eng Data 60:3392–3396. https://doi.org/10.1021/acs.jced.5b00652
Sheng Q, Da Silveira KC, Tian W et al (2017) Simultaneous hydrate and corrosion inhibition with modified poly(vinyl caprolactam) polymers. Energy Fuels 31:6724–6731. https://doi.org/10.1021/acs.energyfuels.7b00525
Sivabalan V, Hasnor N, Lal B, Kassim Z, Shah MA (2020a) Tetraethylammonium acetate and tetraethylammonium bromide-based deep eutectic solvents as thermodynamic CO2 gas hydrate inhibitors. Appl Sci 10(19):6794
Sivabalan V, Hassan NA, Qasim A et al (2020b) Density measurement of aqueous tetraethylammonium bromide and tetraethylammonium iodide solutions at different temperatures and concentrations. S Afr J Chem Eng 32:62–67. https://doi.org/10.1016/j.sajce.2020.03.002
Sloan D, Koh C, Ak S et al (2010) Natural gas hydrates in flow assurance. Gulf Professional Publishing, Housto
Sulaimon AA, Masri AN, Ahmad Shahpin MH et al (2020) Synthesis of dihydrogen phosphate-based ionic liquids: experimental and COSMO-RS based investigation for methane hydrate inhibition. J Mol Liq 319:114092. https://doi.org/10.1016/j.molliq.2020.114092
Sum AK, Koh CA, Sloan ED (2009) Clathrate hydrates: from laboratory science to engineering practice. Ind Eng Chem Res 48:7457–7465. https://doi.org/10.1021/ie900679m
Sundramoorthy JD, Sabil KM, Lal B, Hammonds P (2015) Catastrophic crystal growth of clathrate hydrate with a simulated natural gas system during a pipeline shut-in condition. Cryst Growth Des 15:1233–1241. https://doi.org/10.1021/cg501626h
Sundramoorthy JD, Hammonds P, Lal B, Phillips G (2016) Gas hydrate gas hydrate equilibrium measurement and observation of gas hydrate dissociation with/without a KHI. Procedia Eng 148:870–877. https://doi.org/10.1016/j.proeng.2016.06.476
Talaghat MR (2014) Enhancement of the performance of modified starch as a kinetic hydrate inhibitor in the presence of polyoxides for simple gas hydrate formation in a flow mini-loop apparatus. J Nat Gas Sci Eng 18:7–12. https://doi.org/10.1016/j.jngse.2014.01.013
Tariq M, Rooney D, Othman E et al (2014) Gas hydrate inhibition: a review of the role of ionic liquids. Ind Eng Chem Res 53:17855–17868. https://doi.org/10.1021/ie503559k
Tariq M, Connor E, Thompson J et al (2016) Doubly dual nature of ammonium-based ionic liquids for methane hydrates probed by rocking-rig assembly. RSC Adv 6:23827–23836. https://doi.org/10.1039/c6ra00170j
Tumba K, Reddy P, Naidoo P et al (2011) Phase equilibria of methane and carbon dioxide clathrate hydrates in the presence of aqueous solutions of tributylmethylphosphonium methylsulfate ionic liquid. J Chem Eng Data 56:3620–3629. https://doi.org/10.1021/je200462q
Vavik D (2020) Implications of gas hydrates in drilling and completion: a scientific root cause analysis of the deepwater horizon disaster
Wang Z, Zhao Y, Zhang J et al (2018) Flow assurance during deepwater gas well testing: Hydrate blockage prediction and prevention. J Pet Sci Eng 163:211–216. https://doi.org/10.1016/j.petrol.2017.12.093
Wang C, Liu Y, Yu C et al (2021) Dynamic risk analysis of offshore natural gas hydrates depressurization production test based on fuzzy CREAM and DBN-GO combined method. J Nat Gas Sci Eng 91:103961. https://doi.org/10.1016/j.jngse.2021.103961
Xiao C, Adidharma H (2009) Dual function inhibitors for methane hydrate. Chem Eng Sci 64:1522–1527. https://doi.org/10.1016/j.ces.2008.12.031
Xiao C, Wibisono N, Adidharma H (2010) Dialkylimidazolium halide ionic liquids as dual function inhibitors for methane hydrate. Chem Eng Sci 65:3080–3087. https://doi.org/10.1016/j.ces.2010.01.033
Xiong L, Li X, Wang Y, Xu C (2012) Experimental study on methane hydrate dissociation by depressurization in porous sediments. Energies 5:518–530. https://doi.org/10.3390/en5020518
Yaqub S, Lal B, Partoon B, Mellon NB (2018) Investigation of the task oriented dual function inhibitors in gas hydrate inhibition: a review. Fluid Phase Equilib 477:4057. https://doi.org/10.1016/j.fluid.2018.08.015
Zare M, Haghtalab A, Ahmadi AN, Nazari K, Mehdizadeh A (2015) Effect of imidazolium based ionic liquids and ethylene glycol monoethyl ether solutions on the kinetic of methane hydrate formation. J Mol Liq 204:236–242. https://doi.org/10.1016/j.molliq.2015.01.034
Acknowledgements
Authors would acknowledge the support by MyPAIR-PHC-Hibiscus Grant [MyPAIR/1/2020/STG05/UNIM//1] and Fundamental Research Grant Scheme, Malaysia [FRGS/1/2019/STG05/UNIM/02/2]. The authors acknowledged support from the Hibiscus Hubert Curien Partnership founded by the French Ministry of Foreign Affairs (Ministère de l'Europe et des Affaires étrangères) and the French Ministry of Research (Ministère de l’Enseignement Supérieur, de la rechercher et de l'innovation) Author would also like to thank Universiti Teknologi Petronas, Bandar Seri Iskandar, Malaysia, for supporting the research work. Authors are also thankful to the Asia Pacific University of Technology & Innovation, Malaysia, for the technical support. The authors would also like to acknowledge UCSI University Research and Innovation Grant [REIG-FAS-2020/028] for supporting the research work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ul Haq, I., Qasim, A., Lal, B. et al. Ionic liquids for the inhibition of gas hydrates. A review. Environ Chem Lett 20, 2165–2188 (2022). https://doi.org/10.1007/s10311-021-01359-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-021-01359-9