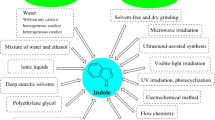
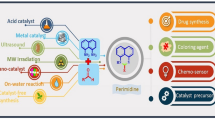
Abstract
Heterocyclic moieties are basic skeletons for 80% of commercial medicines according to the US retail market in 2014–2015, yet many actual synthetic methods are not sustainable, calling for eco-friendly strategies. For instance, microwave-assisted synthesis produces molecules rapidly in excellent yields using less energy. Similarly, in nanoparticle-catalysed synthesis, the use of metal-impregnated nanoparticles provides benefits such as recyclability of catalysts, excellent yields and short reaction times. Other sustainable methods include solvent-free synthesis, ionic liquid-supported synthesis, organic synthesis in water, sonochemical synthesis and combinatorial synthesis. Here, we review the application of microwave irradiation for organic synthesis, organic synthesis in water and solvent-free synthesis in a combined manner. We present the synthesis of complex heterocyclic molecules using nanoparticles as catalysts. We emphasize green aspects of synthetic procedures.












































































Similar content being viewed by others
Abbreviations
- DCC:
-
N,N-dicyclohexylcarbodimide
- DMAP:
-
Dimethylaminopyridine
- Na2CO3 :
-
Sodium carbonate
- DIPEA:
-
N,N-diisopropylethylamine
- Pd BNP:
-
Palladum binaphthyl
- DMSO:
-
Dimethyl sulphoxide
- LiOBu:
-
Lithium tert-butoxide
- DABCO:
-
1,4-Diazabicyclooctane
- CuSO4 :
-
Copper(II)sulphate
- FeCl2 :
-
Iron(II) chloride
- NiCl2 :
-
Nickel(II) chloride
- AgNO3 :
-
Silver nitrate
- DMF:
-
Dimethylformamide
- AgI:
-
Silver iodide
- PVP:
-
Polyvinylpyrrolidone
- ICP-AES:
-
Inductively coupled plasma atomic emission spectroscopy
- SDS:
-
Sodium lauryl sulphate
- NaOH:
-
Sodium hydroxide
- LiCl:
-
Lithium chloride
- SiO2 :
-
Silicon dioxide
- HClO4 :
-
Perchloric
- TEOS:
-
Tetraethylorthosilicate
- DDBSA:
-
Dodecyl benzenesulphonic acid
- TEM:
-
Transmission electronic microscopy
- PEG:
-
Polyethylene glycol
- MeOH:
-
Methanol
- KCN:
-
Potassium cyanide
- DBU:
-
Diazabicycloundecene
- CNBr:
-
Cyanogen bromide
- NaOMe:
-
Sodium methoxide
- Al2O3 :
-
Aluminium oxide
- TFA:
-
Trifluoroacetic acid
- DCM:
-
Dichloromethane
- CuBr:
-
Copper(I)bromide
- VEGFR:
-
Vascular endothelial growth factor receptor
- K2CO3 :
-
Potassium carbonate
- Cs2CO3 :
-
Cesium carbonate
- t-BuOLi:
-
Lithium tertiary butoxide
- CuCl:
-
Copper(I)chloride
- NBS:
-
N-Bromosuccinamide
- Et3N:
-
Triethylamine
- t-BuOK:
-
Potassium tertiary butoxide
- CuI:
-
Copper iodide
- AgOTf:
-
Silver trifluoromethanesulphonate
- H2O:IPA:
-
Water/isopropanol
- Pd(OAc)2 :
-
Palladium(II) acetate
- Fe(CO)5 :
-
Iron pentacarbonyl
- CO:
-
Carbon monoxide
- HCOONH4 :
-
Ammonium formate
- EtOH:
-
Ethanol
- TSILs:
-
Task-specific ionic liquids
- CH3CN:
-
Acetonitrile
- HMDS:
-
Hexamethyldisilazane
- TMSOTf:
-
Trimethylsilyl trifluoromethanesulphonate
- MCR:
-
Multicomponent reaction
- Fe3O4 :
-
Iron oxide
- ZnO:
-
Zinc oxide
- TiO2 :
-
Titanium dioxide
- CuO:
-
Copper (II) oxide
- THF:
-
Tetrahydrofuran
- DTTB:
-
5,7-Dichloro-4-(2,4,5-trichlorophenoxy)-2-(trifluoromethyl)-1H-benzimidazole
- NH2OH·HCl:
-
Hydroxylamine hydrochloride
- MW:
-
Microwave
- Ar:
-
Aryl group
- Et:
-
Ethyl group
- CuI:
-
Copper Iodide
- K2CO3 :
-
Potassium carbonate
- TBAB:
-
Tetrabutylammonium bromide
- EtOH:
-
Ethanol
- DMTMM:
-
4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride
- H2SO4 :
-
Sulphuric acid
- MgSO4 :
-
Magnesium sulphate
- Sc(OTf)3 :
-
Scandium(III) triflate
- CH3ONa:
-
Sodium methoxide
- NaH:
-
Sodium hydride
- NaOtBu:
-
Sodium tertiary butoxide
- CuSO4·5H2O:
-
Copper(II) sulphate pentahydrate
- DMS:
-
Dimethylsulphate
- Cu(OTf)2 :
-
Copper(II) trifluoromethanesulphonate
- InCl3 :
-
Indium chloride
- EDC:
-
1,2-Dichloroethane
- Cu(OAc)2 :
-
Copper(II) acetate
- POCl3 :
-
Phosphoryl chloride
- NaBF4 :
-
Sodium tetrafluoroborate
- DMF-DMA:
-
N,N-dimethylformamide dimethyl acetal
- N2H4 :
-
Hydrazine
- MPa:
-
Megapascal
- Au:
-
Gold
- Fe2O3 :
-
Iron(III) oxide
- CuNP:
-
Copper nanoparticles
- PS-PdONPs:
-
Polystyrene palladium nanoparticles
- Cu2O:
-
Copper(I)oxide
- CuFe2O4 :
-
Copper iron oxide
- Ag:
-
Silver
- Ru:
-
Ruthenium
- TDSN:
-
Triazine dendrimer stabilized magnetic polymer
- Bi:
-
Bismuth
- Pd-BNP:
-
Palladium binapthyl
- KI:
-
Potassium iodide
- KF:
-
Potassium fluoride
- CP:
-
Clinoptilolite
- NaOAc:
-
Sodium acetate
- Phen:
-
1,10-Phenanthroline
- ee:
-
Enantiomeric excess
- NMR:
-
Nuclear magnetic resonance
- h:
-
Hour
- min:
-
Minutes
- COD:
-
1,5-Cyclooctadiene
- COT:
-
1,3,5-Cyclooctatriene
- Au/TiO2 :
-
Gold/Titanium dioxide
- Fe(NO3)3 :
-
Iron(III) nitrate
- Cu(NO3)2 :
-
Copper(II) nitrate
- Pd:
-
Palladium
- Pt:
-
Platium
- KAuCl4 :
-
Potassium gold(III) chloride
- NaHCO3 :
-
Sodium bicarbonate
- PdCl2 :
-
Palladium(II) chloride
- Pd(dba)2 :
-
Bis(dibenzylideneacetone)palladium(0
References
Acosta P, Insuasty B, Ortiz A, Abonia R, Sortino M, Zacchino SA, Quiroga J (2016) Solvent-free microwave-assisted synthesis of novel pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidines with potential antifungal activity. Arabian J Chem 9:481–492. https://doi.org/10.1016/j.arabjc.2015.03.002
Adam D (2003) Out of the kitchen. Nature 421:571–572. https://doi.org/10.1038/421571a
Ali I, Hassan YE, Ashraf G (2005) Enantioselective toxicity and carcinogenesis. Cur Pharm Anal 1:109–125. https://doi.org/10.2174/1573412052953328
Ali I, Kishwar S, Ashanul H, Azzouny AE (2010) Natural products: Human-friendly anti-cancer medications. Egyp Pharm J 9:133–179. https://doi.org/10.1111/j.1751-7915.2010.00221.x
Ali I, Kishwar S, Aboul HY, Ashraf R (2011) Social aspects of cancer genesis. Can Ther. 8:6–14
Ali I, Waseem AW, Kishwar S, Ashanul H (2012a) Thalidomide: a banned drug resurged into future anticancer drug. Curr Drug Ther 7:13–23. https://doi.org/10.2174/157488512800389164
Ali I, Waseem AW, Amber K, Ashanul H, Aijaz A, Kishwar S, Nikhat M (2012b) Synthesis and synergistic antifungal activities of a pyrazoline based ligand and its copper(II) and nickel(II) complexes with conventional antifungals. Microb Pathogen 53:66–73. https://doi.org/10.1016/j.micpath.2012.04.005
Ali I, Ashanul H, Waseem AW, Kishwar S (2013a) Analyses of anticancer drugs by capillary electrophoresis. Biomed Chromatogr 27:1296–1311. https://doi.org/10.1002/bmc.2953
Ali I, Kishwar S, Diana W, Ashanul H (2013b) Synthesis, DNA binding, hemolytic, and anti-cancer assays of curcumin I-based ligands and their ruthenium(III) complexes. Med Chem Res 22:1386–1398. https://doi.org/10.1007/s00044-012-0133-8
Ali I, Waseem AW, Kishwar S, Diana W (2013c) Syntheses, DNA binding and anticancer profiles of L-Glutamic acid ligand and it’s copper(II) and ruthenium(III) complexes. Med Chem 9:11–21. https://doi.org/10.2174/157340613804488297
Ali I, Waseem AW, Kishwar S, Ming FH (2013d) Design and synthesis of thalidomide based dithiocarbamate Cu(II), Ni(II) and Ru(III) complexes as anticancer agents. Polyhedron 56:134–143. https://doi.org/10.1016/j.poly.2013.03.056
Ali I, Waseem AW, Kishwar S, Ming FH (2014) Anticancer metallodrugs of glutamic acid sulphonamides: in silico, DNA binding, hemolysis and anticancer studies. RSC Adv 4:29629–29641. https://doi.org/10.1039/C4RA02570A
Ali I, Mohammad NL, Haasan Y, Aboul E (2017) Imidazoles as potential anticancer agents. Med Chem Commun 8:1742–1773. https://doi.org/10.1039/C7MD00067G
Ali I, Sofi DM, Ming FH, Zeid AA, Abdulrahman A (2018) Facile synthesis of indole heterocyclic compounds based micellar nano anti-cancer drugs. RSC Adv 8:37905. https://doi.org/10.1039/C8RA07060A
Ali I, Mohammad NL, Zeid AA, Ahmad YB, Abdullah GA (2019) Spectroscopic and in silico DNA binding studies on the interaction of some new N-substituted rhodamines with calf-thymus DNA: In vitro anticancer activities. Antican Agents Med Chem 9:425–433. https://doi.org/10.2174/1871520618666181002131125
Anastas PT, Eghbali N (2010) Green chemistry: principles and practice. Chem Soc Rev 39:301–312. https://doi.org/10.1039/B918763B
Anastas PT, Warner JC (1998) Green Chemistry: theory and practice. Oxford University Press, Oxford
Asadi B, Landarani-Isfahani A, Mohammadpoor-Baltork I, Tangestaninejad S, Moghadam M, Mirkhani V, Rudbari HA (2017) Diastereoselective synthesis of symmetrical and unsymmetrical tetrahydropyridines catalysed by bi(iii) immobilized on triazine dendrimer stabilized magnetic nanoparticles. ACS Comb Sci 19:356–364. https://doi.org/10.1021/acscombsci.6b00180
Astruc D (2020) Introduction: nanoparticles in catalysis. Chem Rev 120:461–463. https://doi.org/10.1021/acs.chemrev.8b00696
Atsushi O, Takuto T, Ryohei F, Kanako I, Takahiro K, Koichi T, Osamu S, Ryoki N (2011) Linear polystyrene-stabilized palladium nanoparticles-catalyzed C-C coupling reaction in water. J Org Chem 76:4052–4060. https://doi.org/10.1021/jo200485q
Balou J, Khalizadeh MA, Zareyee D (2019) An efficient and reusable nano catalyst for the synthesis of benzoxanthene and chromene derivatives. Sci Rep 9:3605–3613. https://doi.org/10.1038/s41598-019-40431-x
Balwe SG, Shinde VV, Rokade AA, Park SS, Jeong YT (2017) Green synthesis and characterization of silver nanoparticles (Ag NPs) from extract of plant Radix Puerariae: An efficient and recyclable catalyst for the construction of pyrimido[1,2-b]indazole derivatives under solvent-free conditions. Catal Commun 99:121–126. https://doi.org/10.1016/j.catcom.2017.06.006
Bariwal JB, Ermolatev DS, Glasnov TN, Van Hecke K, Mehta VP, Meervelt LV, Kappe CO, Van der Eycken EV (2010) Diversity-oriented synthesis of dibenzoazocines and dibenzoazepines via a microwave-assisted intramolecular A3-coupling reaction. Org Lett 12:2774–2777. https://doi.org/10.1021/ol1008729
Barve IJ, Thikekar TU, Sun CM (2017) Silver(I)-catalyzed regioselective synthesis of triazole fused-1,5- benzoxazocinones. Org Lett 19:2370–2373. https://doi.org/10.1021/acs.orglett.7b00907
Baumann M, Baxendale IR (2013) An overview of the synthetic routes to the best selling drugs containing 6-membered heterocycles. Beilstein J Org Chem 9:2265–2319. https://doi.org/10.3762/bjoc.9.265
Bazgir A, Hosseini G, Ghahremanzadeh R (2013) Copper ferrite nanoparticles: an efficient and reusable nanocatalyst for a green one-pot, three-component synthesis of spirooxindoles in water. ACS Comb Sci 15:530–534. https://doi.org/10.1021/co400057h
Bogdal D, Warzala M (2000) Microwave-assisted preparation of benzo[b]furans under solventless phase-transfer catalytic conditions. Tetrahedron 56:8769–8773. https://doi.org/10.1016/S0040-4020(00)00818-8
Boominathan M, Pugazhenthiran N, Nagaraj M, Muthusubramanian S, Murugesan S, Bhuvanesh N (2013) Nanoporous titania-supported gold nanoparticle-catalyzed green synthesis of 1,2,3-triazoles in aqueous medium. ACS Sustain Chem Eng 1:1405–1411. https://doi.org/10.1021/sc400147r
Castillo JC, Tigreros A, Portilla J (2018) 3-Formylpyrazolo[1,5-a]pyrimidines as key intermediates for the preparation of functional fluorophores. J Org Chem 83:10887–10897. https://doi.org/10.1021/acs.joc.8b01571
Chan CK, Lai CY, Wang CC (2020) TMSOTf-Catalyzed synthesis of substituted quinazolines using hexamethyldisilazane as nitrogen source under neat and microwave irradiation. Org Biol Chem 18:7201–7212. https://doi.org/10.1039/D0OB01507E
Chanda A, Fokin VV (2009) Organic synthesis “On Water.” Chem Rev 109:725–748. https://doi.org/10.1021/cr800448q
Chanda K, Kuo J, Chen CH, Sun CM (2009) Enantioselective synthesis of benzimidazolyl quinoxalinones on soluble polymer support using focused microwave irradiation. J Comb Chem 11:252–260. https://doi.org/10.1021/cc800137p
Chanda K, Maiti B, Yellol GS, Chien MH, Kuo ML, Sun CM (2011) Polymer supported synthesis of novel benzoxazole linked benzimidazoles under microwave conditions: in vitro evaluation of VEGFR-3 kinase inhibition activity. Org Biomol Chem 9:1917–1926. https://doi.org/10.1039/C0OB00547A
Chanda K, Rej S, Huang MH (2013a) Facet-dependent catalytic activity of Cu2O nanocrystals in the one-pot synthesis of 1,2,3-triazoles by multicomponent click reactions. Chem Eur J 19:16036–16043. https://doi.org/10.1002/chem.201302065
Chanda K, Rej S, Huang MH (2013b) Investigation of facet effects on the catalytic activity of Cu2O nanocrystals for efficient regioselective synthesis of 3,5-disubstituted isoxazoles. Nanoscale 5:12494–12501. https://doi.org/10.1039/C3NR03790H
Chang WJ, Kulkarni MV, Sun CM (2006) Traceless and stereoselective synthesis of tetrahydro-β-carbolinethiohydantoins by microwave irradiation. J Comb Chem 8:141–144. https://doi.org/10.1021/cc050098j
Chen LH, Hsiao YS, Yellol GS, Sun CM (2011a) Microwave promoted simple, efficient and regioselective synthesis of trisubstituted imidazo[1,2-a]benzimidazoles on soluble support. ACS Comb Sci 13:112–119. https://doi.org/10.1021/co1000037
Chen CH, Yellol GS, Lin PT, Sun CM (2011b) Base-catalyzed povarov reaction: an unusual [1,3] sigmatropic rearrangement to dihydropyrimidobenzimidazoles. Org Lett 13:5120–5123. https://doi.org/10.1021/ol201985p
Dai WM, Xuan W, Chen M (2005) Microwave-assisted one-pot regioselective synthesis of 2-alkyl-3,4-dihydro-3-oxo-2H-1,4-benzoxazines. Tetrahedron 61:6879–6885. https://doi.org/10.1016/j.tet.2005.04.072
Dalvi PB, Lin SF, Paike V, Sun CM (2015) Microwave-assisted multicomponent synthesis of dihydroquinoxalinones on soluble polymer support. ACS Comb Sci 17:421–425. https://doi.org/10.1021/acscombsci.5b00053
Dhole S, Selvaraju M, Maiti B, Chanda K, Sun CM (2015) Microwave controlled reductive cyclization: a selective synthesis of novel benzimidazole-alkyloxypyrrolo[1,2-a]quinoxalinones. ACS Comb Sci 17:310–316. https://doi.org/10.1021/acscombsci.5b00010
Diaz-Ortiz A, Carrillo JR, Cossio FR, Gómez-Escalonilla MJ, de la Hoz A, Moreno A, Prieto P (2000) Synthesis of pyrazolo[3,4-b]pyridines by cycloaddition reactions under microwave irradiation. Tetrahedron 56:1569–1577. https://doi.org/10.1016/S0040-4020(00)00059-4
Donets P, Van der Eycken EV (2007) Synthesis of ring-expanded aza-analogues of bisbenzocyclooctadiene lignan lactones. QSAR Comb Sci 26:1239–1242. https://doi.org/10.1002/qsar.200740092
Faisal M (2020) Sonochemical protocol for solvent-free organic synthesis. In: Boddula R, Asiri AM (eds) Green sustainable process for chemical and environmental engineering and science: sonochemical organic synthesis. Elsevier, Amsterdam, pp 113–139. https://doi.org/10.1016/C2018-0-05312-8
Francisco A, Yanina M, Gabriel R, Yus M (2011) Multicomponent click synthesis of 1,2,3-triazoles from epoxides in water catalyzed by copper nanoparticles on activated carbon. J Org Chem 76:8394–8405. https://doi.org/10.1021/jo2016339
Ganji P, van Leeuwen PWNM (2017) Phosphine Supported Ruthenium Nanoparticle Catalyzed Synthesis of Substituted Pyrazines and Imidazoles from α-Diketones. J Org Chem. https://doi.org/10.1021/acs.joc.6b03032
Giacomelli G, Lidia DL, Andrea PA (2003) A method for generating nitrile oxides from nitroalkanes: a microwave assisted route for isoxazoles. Tetrahedron 59:5437–5440. https://doi.org/10.1021/acssuschemeng.5b00689
Giernoth R (2010) Task-specific ionic liquids. Angew Chem Int Ed 49:2834–2839
Guo X, Wang L, Hu J, Zhang M (2018) CuI nanoparticle-catalyzed synthesis of tetracyclic benzo[e]benzo[4,5]imidazo[1,2-c][1,3]thiazin-6- imine heterocycles by SNAr-type C-S, C–N bond formation from isothiocyanatobenzenes and benzimidazoles. RSC Adv 8:22259–22267. https://doi.org/10.1039/C8RA02552E
Horváth IT (2018) Introduction: sustainable chemistry. Chem Rev 118:369–371. https://doi.org/10.1021/acs.chemrev.7b00721
Hsiao YS, Yellol GS, Chen LH, Sun CM (2010) Multidisciplinary synthetic approach for rapid combinatorial library synthesis of triaza-fluorenes. J Comb Chem 12:723–732. https://doi.org/10.1021/cc1000902
Hsiao YS, Narhe BD, Chang YS, Sun CM (2013) One-pot, two-step synthesis of imidazo[1,2-a]benzimidazoles via a multicomponent [4+1] cycloaddition reaction. ACS Comb Sci 15:551–555. https://doi.org/10.1021/co400075z
Hsu WS, Tsai MH, Barve IJ, Yellol GS, Sun CM (2017) Synthesis of aminofuran-linked benzimidazoles and cyanopyrrole fused benzimidazoles by condition-based skeletal divergence. ACS Comb Sci 19:492–499. https://doi.org/10.1021/acscombsci.7b00052
Ibrahim HM, Behbehani H, Arafa WAA (2020) A facile, practical and metal-free microwave-assisted protocol for mono- and bis-[1,2,4]triazolo[1,5-a]pyridines synthesis utilizing 1-amino-2- imino-pyridine derivatives as versatile precursors. RSC Adv 10:15554–15572. https://doi.org/10.1039/D0RA02256J
Insuasty D, Abonia R, Insuasty B, Quiroga J, Laali KK, Nogueras M, Cobo J (2017) Microwave-assisted synthesis of diversely substituted quinoline-based dihydropyridopyrimidine and dihydropyrazolopyridine hybrids. ACS Comb Sci 19:555–563. https://doi.org/10.1021/acscombsci.7b00091
Jia D, Li M, Fei Y, Zhang Z, Shou L (2012) One-pot, three-component synthesis of a library of spirooxindolepyrimidines catalyzed by magnetic nanoparticle supported dodecyl benzenesulfonic acid in aqueous media. ACS Comb Sci 14:335–341. https://doi.org/10.1021/co3000264
Jiang B, Feng BM, Wang SL, Tu SJ, Li G (2012) Domino constructions of pentacyclic indeno[2,1-c]quinolines and pyrano[4,3-b]oxepines by [4+1]/[3+2+1]/[5+1] and [4+3] multiple cyclizations. Chem Eur J 18:9823–9826. https://doi.org/10.1002/chem.201201109
Kappe OC (2008) Microwave dielectric heating in synthetic organic chemistry. Chem Soc Rev 37:1127–1129. https://doi.org/10.1039/B803001B
Kumar Y, Matta A, Kumar P, Parmar VS, Van der Eycken EV, Singh BK (2015) Cu(I)-catalyzed microwave-assisted synthesis of 1,2,3-triazole linked with 4-thiazolidinones: a one-pot sequential approach. RSC Adv 5:1628–1639. https://doi.org/10.1039/C4RA12592D
Kusy D, Maniukiewicz W, Błazewska KM (2019) Microwave-assisted synthesis of 3-formyl substituted imidazo[1,2-a] pyridines. Tetrahedron Lett 60:151244. https://doi.org/10.1016/j.tetlet.2019.151244
Laedbeater N (2010) Microwave heating as a tool for sustainable chemistry. CRC Press, Boca Raton
Lee CH, Hsu WS, Chen CH, Sun CM (2013) A telescoping synthesis of chimeric polyheterocycles through a piperidine-mediated multicomponent reaction. Eur J Org Chem. https://doi.org/10.1002/ejoc.201201645
Lee CH, Wu WC, Dangate PS, Shen LC, Chung WS, Sun CM (2015) Skeletally diverse synthesis of innovative [2,1-c]-1,4-oxazepine and [1,4]-quinoxaline systems. ACS Comb Sci 17:623–630. https://doi.org/10.1021/acscombsci.5b00093
Li JJ (2004) Name reactions in heterocyclic chemistry. Wiley Interscience, New Jersey
Lin WH, Wu WC, Selvaraju M, Sun CM (2017) One-pot synthesis of benzazoles and quinazolinones via iron pentacarbonyl mediated carbonylation of aryl iodides under microwave irradiation. Org Chem Front 4:392–397. https://doi.org/10.1039/C6QO00733C
Maiti B, Sun CM (2011) Novel approach towards the synthesis of skeletally diverse benzimidazole-pyrrolo[1,2-a]quinoxaline by SNAr/Pictet–Spengler reaction under focused microwave irradiation. New J Chem 35:1385–1396. https://doi.org/10.1039/C1NJ20153K
Maiti B, Chanda K, Selvaraju M, Tseng CC, Sun CM (2013) Multicomponent solvent-free synthesis of benzimidazolyl imidazo[1,2-a]-pyridine under microwave irradiation. ACS Comb Sci 15:291–297. https://doi.org/10.1021/co400010y
Maleki A, Movahed H, Ravaghi P (2017) Magnetic cellulose/Ag as a novel eco-friendly nanobiocomposite to catalyze synthesis of chromene-linked nicotinonitriles. Carbohydr Polym 156:259–267. https://doi.org/10.1016/j.carbpol.2016.09.002
Mani P, Sharma C, Kumar S, Awasthi SK (2014) Efficient heterogeneous silver nanoparticles catalysed one-pot synthesis of 5-substituted 1H-tetrazoles. J Mol Catal A: Chem 392:150. https://doi.org/10.1016/j.molcata.2014.05.008
Martins MAP, Frizzo CP, Tier AZ, Moreira DN, Zanatta N, Bonacorso HG (2014) Update 1 of: Ionic liquids in heterocyclic synthesis. Chem Rev 114:PR1–PR70. https://doi.org/10.1021/cr500106x
Mason TJ (1997) Ultrasound in synthetic organic chemistry. Chem Soc Rev 26:443–451. https://doi.org/10.1039/CS9972600443
Mayence A, Pietka A, Collins MS, Cushion MT, Tekwani BL, Huang TL, Vanden Eynde JJ (2008) Novel bisbenzimidazoles with antileishmanial effectiveness. Bioorg Med Chem Lett 18:2658–2661. https://doi.org/10.1016/j.bmcl.2008.03.020
Meena DR, Maiti B, Chanda K (2016) Cu(I) catalyzed microwave assisted telescopic synthesis of 3, 5-disubstituted isoxazoles in green media. Tetrahedron Lett 57:5514–5517. https://doi.org/10.1016/j.tetlet.2016.10.109
Morton GC, Salvino JM, Labaudiníere RF, Herpin TF (2000) Novel solid-phase synthesis of 1,5-benzothiazepine-4-one derivatives. Tetrahedron Lett 41:3029–3033. https://doi.org/10.1016/S0040-4039(00)00341-5
Murlykina MV, Morozova AD, Zviagin LM, Sakhno YI, Desenko SM, Chebanov VA (2018) Aminoazole-based diversity-oriented synthesis of heterocycles. Front Chem 6:527. https://doi.org/10.3389/fchem.2018.00527
Nwankwo CB, Hoque MA, Islam MA, Ashraf D (2020) Groundwater constituents and trace elements in the basement aquifers of africa and sedimentary aquifers of Asia: medical hydrogeology of drinking water minerals and toxicants. Earth Syst Environ 4:369–384. https://doi.org/10.1007/s41748-020-00151-z
Obermayer D, Glasnov TN, Kappe CO (2011) Microwave-assisted and continuous flow multistep synthesis of 4-(pyrazol-1-yl)carboxanilides. J Org Chem 76:6657–6669. https://doi.org/10.1021/jo2009824
Padmaja RD, Chanda K (2020) A robust and recyclable ionic liquid-supported copper(II) catalyst for the synthesis of 5-substituted-1H-tetrazoles using microwave irradiation. Res Chem Intermed 46:1307–1317. https://doi.org/10.1007/s11164-019-04035-4
Padmaja RD, Meena DR, Maiti B, Chanda K (2017a) [Cu(phen)(PPh3)2]NO3-catalyzed microwave-assisted green synthesis of 5-substituted 1H-tetrazoles. Res Chem Intermed 43:7365–7374. https://doi.org/10.1007/s11164-017-3080-7
Padmaja RD, Rej S, Chanda K (2017b) Environmentally friendly, microwave-assisted synthesis of 5-substituted 1H-tetrazoles by recyclable CuO nanoparticles via (3+2) cycloaddition of nitriles and NaN3. Chin J Catal 38:1918–1924. https://doi.org/10.1016/S1872-2067(17)62920-6
Padmaja RD, Balamurali MM, Chanda K (2019) One-pot, telescopic approach for the chemoselective synthesis of substituted benzo[e]pyrido/pyrazino/pyridazino[1,2-b][1,2,4]thiadiazine dioxides and their significance in biological systems. J Org Chem 84:11382–11390. https://doi.org/10.1021/acs.joc.9b00869
Panchangam RL, Manickam V, Chanda K (2019) Assembly of fully substituted 2h-indazoles catalysed by Cu2O rhombic dodecahedra and evaluation of anticancer activity. ChemMedChem 14:262–272. https://doi.org/10.1002/cmdc.201800707
Parveen N, Sekar G (2020) Palladium nanoparticle-catalyzed stereoselective domino synthesis of 3-allylidene-2(3h)-oxindoles and 3-allylidene-2(3h)-benzofuranones. J Org Chem 85:4682–4694. https://doi.org/10.1021/acs.joc.9b03397
Peshkov VA, Pereshivko OP, Donets PA, Mehta VP, Van der Eycken EP (2010) Diversity-oriented microwave-assisted synthesis of the 3-benzazepine framework. Eur J Org Chem. https://doi.org/10.1002/ejoc.201000583
Pitt WR, Parry DM, Perry BG, Groom CR (2009) Heteroaromatic rings of the future. J Med Chem 52:2952–2963. https://doi.org/10.1021/jm801513z
Plaquevent JC, Levillain J, Guillen FDR, Malhiac C, Gaumont AC (2008) Ionic liquids: new targets and media for α-amino acid and peptide chemistry. Chem Rev 108:5035–5060. https://doi.org/10.1021/cr068218c
Polo E, Ferrer-Pertuz K, Trilleras J, Quiroga J, Margarita G (2017) Microwave-assisted one-pot synthesis in water of carbonylpyrazolo[3,4-b]pyridine derivatives catalyzed by InCl3 and sonochemical assisted condensation with aldehydes to obtain new chalcone derivatives containing the pyrazolopyridinic moiety. RSC Adv 7:50044–50055. https://doi.org/10.1039/C7RA10127A
Polshettiwar V, Verma RS (2008a) Microwave-assisted organic synthesis and transformations using benign reaction media. Acc Chem Res 41:629–639. https://doi.org/10.1021/ar700238s
Polshettiwar V, Verma RS (2008b) Greener and rapid access to bio-active heterocycles; One-pot solvent-free synthesis of 1,3,4-oxadiazoles and 1,3,4-thiadiazole. Tetrahedron Lett 49:879–883. https://doi.org/10.1016/j.tetlet.2007.11.165
Porco JA Jr, Cong H (2012) Chemical synthesis of complex molecules using nanoparticle catalysis. ACS Catal 2:65–70. https://doi.org/10.1021/cs200495s
Portela-Cubillo F, Scott JS, Walton JC (2007) Microwave-assisted preparations of dihydropyrroles from alkenone O-phenyl oximes. Chem Commun. https://doi.org/10.1039/B712582H
Quiroga J, Alvarado M, Insuasty B, Moreno R (1999) Synthesis of 5 cyanopyrazolo[3,4-b]pyridines in the reaction of 5-amino-3-methyl-1-phenylpyrazole with arylidene derivatives of malonodinitrile and ethyl cyanoacetate. J Heterocyclic Chem 36:311–1316. https://doi.org/10.1002/jhet.5570360533
Rao RN, Chanda K (2020a) Anthology of heterocyclic pharmacophores synthesized under solvent-free conditions: a decade survey. In: Boddula R, Asiri AM (eds) Green sustainable process for chemical and environmental engineering and science: sustainable organic synthesis. Elsevier, Amsterdam, pp 199–222. https://doi.org/10.1016/B978-0-12-819539-0.00008-7
Rao RN, Chanda K (2020b) An assessment study of known pyrazolopyrimidines: chemical methodology and cellular activity. Bioorg Chem. https://doi.org/10.1016/j.bioorg.2020.103801
Rao RN, Chanda K (2021) An expeditious microwave assisted one-pot sequential route to pyrido fused imidazo[4,5-c]quinolines in green media. New J Chem 45:3280–3289. https://doi.org/10.1039/D0NJ05835A
Rao RN, Maiti B, Chanda K (2017) Application of pictet-spengler reaction to indole-based alkaloids containing tetrahydro-β-carboline scaffold in combinatorial chemistry. ACS Comb Sci 19:199–228. https://doi.org/10.1021/acscombsci.6b00184
Rao RN, Balamurali MM, Maiti B, Thakuria R, Chanda K (2018) Efficient access to imidazo[1,2-a]pyridines\/pyrazines\/pyrimidines via catalyst-free annulation reaction under microwave irradiation in green solvent. ACS Comb Sci 20:164–171. https://doi.org/10.1021/acscombsci.7b00173
Rao RN, Maiti B, Chanda K (2020) Organic synthesis on ionic liquid support: a new strategy for the liquid-phase organic synthesis (LPOS). In: Asiri AM, Kanchi S (eds) Green sustainable process for chemical and environmental engineering and science: ionic liquids as green solvents. Elsevier, Amsterdam, pp 49–104. https://doi.org/10.1016/B978-0-12-817386-2.00003-2
Rej S, Chanda K, Chun CY, Huang MH (2014) Control of regioselectivity over gold nanocrystals of different surfaces for the synthesis of 1,4-disubstituted triazole through the click reaction. Chem Eur J 20:15991–15997. https://doi.org/10.1002/chem.201403958
Safaei-Ghomi J, Ghasemzadeh MA (2015) An efficient multi-component synthesis of 14-aryl-14H-dibenzo[a, j]xanthene derivatives by AgI nanoparticles. J Saudi Chem Soc 19:642–649. https://doi.org/10.1016/j.jscs.2012.05.007
Saha D, Wadhwa P, Sharma A (2015) A sequential synthetic strategy towards unexplored dibenzo[b, f][1,4]thiazepine carboxamides: copper catalysed C-S cyclisation followed by Ugi type 3CC cascade. RSC Adv 5:33067–33076. https://doi.org/10.1039/C5RA04175A
Saha R, Arunprasath D, Sekar D (2018) Phosphine-free and reusable palladium nanoparticles-catalyzed domino strategy: synthesis of indanone derivatives. J Org Chem 83:4692–4702. https://doi.org/10.1021/acs.joc.8b00463
Saikia AA, Rao RN, Das S, Jena S, Rej S, Maiti B, Chanda K (2020a) Sequencing [3+ 2]-cycloaddition and multicomponent reactions: a regioselective microwave-assisted synthesis of 1, 4-disubstituted 1, 2, 3-triazoles using ionic liquid supported Cu (II) precatalysts in methanol. Tetrahedron Lett 61:152273. https://doi.org/10.1016/j.tetlet.2020.152273
Saikia AA, Rao RN, Maiti B, Balamurali MM, Chanda K (2020b) Diversity-oriented synthesis of thiazolidine-2-imines via microwave-assisted one-pot, telescopic approach and its interaction with biomacromolecules. ACS Comb Sci 22:630–640. https://doi.org/10.1021/acscombsci.0c00083
Savva I, Kalogirou AS, Achilleos M, Vasile E, Koutentis PA, Krasia CT (2016) Evaluation of PVP/Au nanocomposite fibers as heterogeneous catalysts in indole synthesis. Molecules 21:1218–1232. https://doi.org/10.3390/molecules21091218
Shanmugasundaram M, Manikandan S, Raghunathan R (2002) High chemoselectivity in microwave accelerated intramolecular domino Knoevenagel hetero Diels-Alder reactions-an efficient synthesis of pyrano[3-2c]coumarin frameworks. Tetrahedron 58:997–1003. https://doi.org/10.1016/S0040-4020(01)01076-6
Soh YH, Lam Y (2010) Microwave-assisted synthesis of substituted 2-(benzylthio)imidazo[1,2a]pyrimidin-5-ones. J Comb Chem 12:286–291. https://doi.org/10.1021/cc900176x
Tang L, Guo X, Yang Y, Zha Z, Wang Z (2014) Gold nanoparticles supported on titanium dioxide: an efficient catalyst for highly selective synthesis of benzoxazoles and benzimidazoles. Chem Commun 50:6145–6147. https://doi.org/10.1039/C4CC01822B
Taylor AP, Robinson RR, Fobian YM, Blakemore DC, Jones LH, Fadeyi O (2016) Modern advances in heterocyclic chemistry in drug discovery. Org Biol Chem 14:6611–6637. https://doi.org/10.1039/C6OB00936K
Tsai YH, Chanda K, Chu YT, Chiu CY, Huang MH (2014) Direct formation of small Cu2O nanocubes, octahedra, and octapods for efficient synthesis of triazoles. Nanoscale 6:8704–8709. https://doi.org/10.1039/C4NR02076F
Verma RS (1999) Solvent-free organic synthesis using supported reagents and microwave irradiation. Green Chem 1:43–65. https://doi.org/10.1039/A808223E
Verma RS (2001) Solvent-free accelerated organic syntheses using microwaves. Pure Appl Chem 73:193–198. https://doi.org/10.1351/pac200173010193
Wang SL, Cheng C, Wu FY, Jiang B, Shi F, Tu SJ, Rajale T, Li G (2011a) Microwave-assisted multi-component reaction in water leading to highly regioselective formation of benzo[f]azulen-1-ones. Tetrahedron 67:4485–4493. https://doi.org/10.1016/j.tet.2011.05.002
Wang SL, Wu FY, Cheng C, Zhang G, Liu YP, Jiang B, Shi F, Tu SJ (2011b) Multicomponent synthesis of poly-substituted benzo[a]pyrano-[2,3-c]phenazine derivatives under microwave heating. ACS Comb Sci 13:135–139. https://doi.org/10.1021/co1000376
Wu CY, Sun CM (2002) Parallel synthesis of 1,2,3,4-tetrahydro-β-carbolines using microwave irradiation. Synlett 10:1709–1711. https://doi.org/10.1038/nprot.2007.23
Wu CH, Sun CM (2006) Parallel synthesis of amino bis-benzimidazoles by multistep microwave irradiation. Tetrahedron Lett 47:2601–2604. https://doi.org/10.1016/j.tetlet.2006.02.015
Wu TY, Dhole S, Selvaraju M, Sun CM (2018) Regioselective synthesis of pyranone-fused indazoles via reductive cyclization and alkyne insertion. ACS Comb Sci 20:156–163. https://doi.org/10.1021/acscombsci.7b00170
Yamane Y, Liu X, Hamasaki A, Ishida T, Haruta M, Yokoyama T, Tokunaga M (2009) One-pot synthesis of indoles and aniline derivatives from nitroarenes under hydrogenation condition with supported gold nanoparticles. Org Lett 11:5162–5165. https://doi.org/10.1021/ol902061j
Yang Z, Hao WJ, Xu HW, Wang SL, Jiang B, Li G, Tu SJ (2015) Base-promoted transannulation of heterocyclic enamines and 2,3-epoxypropan-1-ones: regio-and stereoselective synthesis of fused pyridines and pyrroles. J Org Chem 80:2781–2789. https://doi.org/10.1021/acs.joc.5b00067
Yeh WP, Chang WJ, Sun ML, Sun CM (2007) Microwave-assisted traceless synthesis of hydantion-fused β-carboline scaffold. Tetrahedron 63:11809–11816. https://doi.org/10.1016/j.tet.2007.09.054
Zhang ZH, Hong YL, Shu HY, Jian WG (2010) Synthesis of 2,3-dihydroquinazolin-4(1h)-ones by three-component coupling of isatoic anhydride, amines, and aldehydes catalyzed by magnetic fe3o4 nanoparticles in water. J Comb Chem 12:643–646. https://doi.org/10.1021/cc100047j
Zhang XY, Yang ZW, Chen Z, Wang J, Yang DL, Shen Z, Hu LL, Xie JW, Zhang J, Cui HL (2016) Tandem copper-catalyzed propargylation/alkyne azacyclization/ isomerization reaction under microwave irradiation: synthesis of fully substituted pyrroles. J Org Chem 81:1778–1785. https://doi.org/10.1021/acs.joc.5b02429
Acknowledgements
The authors acknowledge the Chancellor and Vice Chancellor of VIT University for delivering opportunity to carry out this study. The authors thank VIT for providing “VIT SEED GRANT” for carrying out this research work. Kaushik Chanda thanks CSIR-Govt. of India for funding through Grant No. 01(2913)/17/EMR-II.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nishanth Rao, R., Jena, S., Mukherjee, M. et al. Green synthesis of biologically active heterocycles of medicinal importance: a review. Environ Chem Lett 19, 3315–3358 (2021). https://doi.org/10.1007/s10311-021-01232-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-021-01232-9