Abstract
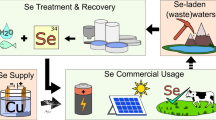
Selenium (Se) has caused several ecological disasters due to its toxicity and bioaccumulation along trophic networks. Industrial activities that process fossil fuels and mineral ores, such as electricity generation, metal extraction and oil refining, produce wastewaters containing selenium. Currently, these wastewaters are insufficiently treated before being discharged into the environment. Several environmental biotechnological processes are used to convert soluble selenium oxyanions, selenite and selenate, to solid elemental selenium, Se(0), because elemental selenium is less toxic. Applying a post-treatment solid–liquid separation step to these biological processes removes and separates Se(0) from the treated effluent. Here, we review the sources of selenium-rich waste streams, and we propose several techniques for the removal and reuse of selenium. The major points are as follows: (1) Biogenic Se(0) has colloidal properties that can be offset by the addition of coagulants, either by dissolving multivalent salts or by electrogenerating the coagulant in situ; (2) recovered biogenic Se(0) is a secondary raw material and (3) biogenic Se(0) can be used for niche applications such as fertilizers and adsorbent for metals. The biological treatment of industrial wastewater containing selenium can be linked with resource recovery as a sound and economic approach to alleviate the demand for this critical element.




Similar content being viewed by others
References
Alfthan G, Eurola M, Ekholm P, Venäläinen ER, Root T, Korkalainen K, Hartikainen H, Salminen P, Hietaniemi V, Aspila P, Aro A (2014) Effects of nationwide addition of selenium to fertilizers on foods and animal and human health in Finland: from deficiency to optimal selenium status of the population. J Trace Elem Med Biol. doi:10.1016/jjtemb201404009
Bebien M, Lagniel G, Garin J, Touati D, Vermeglio A, Labarre J (2002) Involvement of superoxide dismutases in the response of Escherichia coli to selenium oxides. J Bacteriol 184:1556–1564
Buchs B, Evangelou MWH, Winkel LHE, Lenz M (2013) Colloidal properties of nanoparticular biogenic selenium govern environmental fate and bioremediation effectiveness. Environ Sci Technol 47:2401–2407
Chapman PM, Adams WJ, Brooks M, Delos CG, Luoma SN, Maher WA, Ohlendorf HM, Presser TS, Shaw P (2010) Ecological assessment of selenium in the aquatic environments. SETAC Press, Pensacola
DeMoll-Decker H, Macy JM (1993) The periplasmic nitrite reductase of Thauera selenatis may catalyze the reduction of selenite to elemental selenium. Arch Microbiol 160:241–247
Dobias J, Suvorova EI, Bernier-Latmani R (2011) Role of proteins in controlling selenium nanoparticle size. Nanotechnology 22:195605
Espinosa-Ortiz EJ, Gonzalez-Gil G, Saikaly PE, van Hullebusch ED, Lens PNL (2015) Effects of selenium oxyanions on the white-rot fungus Phanerochaete chrysosporium. Appl Environ Microb. doi:10.1007/s00253-014-6127-3
Fellowes JW, Pattrick RA, Green DI, Dent A, Lloyd JR, Pearce CI (2011) Use of biogenic and abiotic elemental selenium nanospheres to sequester elemental mercury released from mercury contaminated museum specimens. J Hazard Mater 189:660–669
Fernandez-Martinez A, Charlet L (2009) Selenium environmental cycling and bioavailability: a structural chemist point of view. Rev Environ Sci Biotechnol 8:81–110
Gates B, Mayers B, Cattle B, Xia Y (2002) Synthesis and characterization of uniform nanowires of trigonal selenium. Adv Funct Mater 12:219–227
Gregory J, Duan J (2001) Hydrolyzing metal salts as coagulants. Pure Appl Chem 73:2017–2026
Harrison GI, Laishley EJ, Krouse HR (1980) Stable isotope fractionation by Clostridium pasteurianum. 3. Effect of SeO3 on the physiology and associated sulfur isotope fractionation during SO3 and SO4 reductions. Can J Microbiol 26:952–958
Harrison GI, Curle C, Laishley EJ (1984) Purification and characterization of an inducible dissimilatory type sulfite reductase from Clostridium pasteurianum. Arch Microbiol 138:72–78
Haygarth PM (1994) Global importance and global cycling of selenium. In: Frankenberger WT, Benson S (eds) Selenium in the environment. Marcel-Dekker, New York, pp 1–28
Hunter WJ, Manter DK (2011) Pseudomonas seleniipraecipitatus sp nov: a selenite reducing γ-Proteobacteria isolated from soil. Curr Microbiol 62:565–569
International Energy Agency (IEA). http://www.iea.org/topics/coal/(2014). Accessed 25 Oct 2014
Jain R, Jordan N, Schild D, van Hullebusch ED, Weiss S, Franzen C, Farges F, Lens PNL (2015a) Adsorption of zinc by biogenic elemental selenium nanoparticles. Chem Eng J 260:855–863
Jain R, Jordan N, Weiss S, Foerstendorf H, Heim K, Kacker R, Hübner R, Kramer H, van Hullebusch ED, Farges F, Lens PNL (2015b) Extracellular polymeric substances (EPS) govern the surface charge of biogenic elemental selenium nanoparticles. Environ Sci Technol. doi:10.1021/es5043063
Janssen AJH, Ruitenberg R, Buisman CJ (2001) Industrial applications of new sulphur biotechnology. Water Sci Technol 44:85–90
Jiang S, Ho CT, Lee JH, Duong HV, Han S, Hur HG (2012) Mercury capture into biogenic amorphous selenium nanospheres produced by mercury resistant Shewanella putrefaciens 200. Chemosphere 87:621–624
Johnson JA, Saboungi ML, Thiyagarajan P, Csencsits R, Meisel D (1999) Selenium nanoparticles: a small-angle neutron scattering study. J Phys Chem B 103:59–63
Johnson NC, Manchester S, Sarin L, Gao Y, Kulaots I, Hurt RH (2008) Mercury vapor release from broken compact fluorescent lamps and in situ capture by new nanomaterial sorbents. Environ Sci Technol 42:5772–5778
Kagami T, Narita T, Kuroda M, Notaguchi E, Yamashita M, Sei K, Soda S, Ike M (2013) Effective selenium volatilization under aerobic conditions and recovery from the aqueous phase by Pseudomonas stutzeri NT-I. Water Res 47:1361–1368
Kessi J, Hanselmann KW (2004) Similarities between the abiotic reduction of selenite with glutathione and the dissimilatory reaction mediated by Rhodospirillum rubrum and Escherichia coli. J Biol Chem 279:50662–50669
Krafft T, Bowen A, Theis F, Macy JM (2000) Cloning and sequencing of the genes encoding the periplasmic-cytochrome B-containing selenate reductase of Thauera selenatis. DNA Seq 10:365–377
Kuroda M, Notaguchi E, Sato A, Yoshioka M, Hasegawa A, Kagami T, Narita T, Yamashita M, Sei K, Soda S, Ike M (2011a) Characterization of Pseudomonas stutzeri NT-I capable of removing soluble selenium from the aqueous phase under aerobic conditions. J Biosci Bioeng 112:259–264
Kuroda M, Yamashita M, Miwa E, Imao K, Fujimoto N, Ono H, Nagano K, Sei K, Ike M (2011b) Molecular cloning and characterization of the srdBCA operon encoding the respiratory selenate reductase complex from the selenate-reducing bacterium Bacillus selenatarsenatis SF-1. J Bacteriol 193:2141–2148
Lemly AD (2002) Symptoms and implications of selenium toxicity in fish: the Belews Lake case example. Aquat Toxicol 57:39–49
Lemly AD (2004) Aquatic selenium pollution is a global environmental safety issue. Ecotoxicol Environ Saf 59:44–56
Li H, Zhang J, Wang T, Luo W, Zhou Q, Jiang G (2008) Elemental selenium particles at nano-size (Nano-Se) are more toxic to Medaka (Oryzias latipes) as a consequence of hyper-accumulation of selenium: a comparison with sodium selenite. Aquat Toxicol 8:251–256
Lortie L, Gould WD, Rajan S, McCready RGL, Cheng KJ (1992) Reduction of selenate and selenite to elemental selenium by a Pseudomonas stutzeri isolate. Appl Environ Microbiol 58:4042–4044
Luoma SN, Johns C, Fisher NS, Steinberg NA, Oremland RS, Reinfelder JR (1992) Determination of selenium bioavailability to a bivalve from particulate and solute pathways. Environ Sci Technol 26:485–491
Macaskie LE, Mikheenko IP, Yong P, Deplanche K, Murray AJ, Paterson-Beedle M, Coker VS, Pearce CI, Cutting R, Pattrick RAD, Vaughan D, van der Laan G, Lloyd JR (2010) Today’s wastes tomorrow’s materials for environmental protection. Hydrometallurgy 104:483–487
Muyzer G, Stams AJM (2008) The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol 6:441–454
North American Metal Council (NAMC) (2010) Review of available technologies for removal of selenium from water. http://www.namcorg/docs/00062756.pdf
Nuttall KL (1987) A model for metal selenide formation under biological conditions. Med Hypotheses 2:217–221
Ohlendorf HM (1989) Bioaccumulation and effects of selenium in wildlife. In: Jacobs LM (ed) Selenium in agriculture and the environment. Soil Science Society of America and American Society of Agronomy, Madison Wisconsin series number 23, pp 133–177. doi:10.2136/sssaspecpub23.c8
Opara A, Peoples MJ, Adams JD, Martin AS (2014) Electro-biochemical reactor (EBR) technology for selenium removal from British Columbia’s coal-mining wastewaters http://www.inotecus/uploads/5/1/2/8/5128573/selenium_removal_coal_mine_water_inotec-sme2014pdf
Pearce CI, Coker VS, Charnock JM, Pattrick RA, Mosselmans JF, Law N, Beveridge TJ, Lloyd JR (2008) Microbial manufacture of chalcogenide-based nanoparticles via the reduction of selenite using Veillonella atypica: an in situ EXAFS study. Nanotechnology 19:1–13
Pickett T, Sonstegard J, Bonkoski B (2008) Using biology to treat selenium. Power Eng 110:140–145
Presser TS, Luoma SN (2006) Forecasting selenium discharges to the San Francisco Bay-Delta estuary: ecological effects of a proposed San Luis drain extension US geological survey professional paper 1646
Purkerson DG, Doblin MA, Bollens SM, Luoma SN, Cutter GA (2003) Selenium in San Francisco Bay zooplankton: potential effects of hydrodynamics and food web interactions. Estuaries 26:956–969
Schlekat CE, Dowdle PR, Lee BG, Luoma SN, Oremland RS (2000) Bioavailability of particle-associated selenium on the bivalve Potamocorbila amuresis. Environ Sci Technol 34:4504–4510
Schmidt JE, Ahring BK (1996) Granular sludge formation in upflow anaerobic sludge blanket (UASB) reactors. Biotechnol Bioeng 49:229–246
Schröder I, Rech S, Krafft T, Macy JM (1997) Purification and characterization of the selenate reductase from Thauera selenatis. J Biol Chem 272:23765–23768
Simmons DB, Wallschlaeger D (2005) A critical review of the biogeochemistry and ecotoxicology of selenium in lotic and lentic environments. Environ Toxicol Chem 24:1331–1343
Staicu LC, van Hullebusch ED, Lens PNL, Pilon-Smits EAH, Oturan MA (2015a) Electrocoagulation of colloidal biogenic selenium. Environ Sci Poll Res. doi:10.1007/s11356-014-3592-2
Staicu LC, Ackerson CJ, Cornelis P, Ye L, Berendsen RL, Hunter WJ, Noblitt SD, Henry CS, Cappa JJ, Montenieri RL, Wong AO, Musilova L, Sura-de Jong M, van Hullebusch ED, Lens PNL, Pilon-Smits EAH (2015b) Pseudomonas moraviensis subsp. stanleyae: a bacterial endophyte capable of efficient selenite reduction to elemental selenium under aerobic conditions. J Appl Microb (in revision)
Staicu LC, van Hullebusch ED, Oturan MA, Ackerson CJ, Lens PNL (2015c) Removal of colloidal biogenic selenium from wastewater. Chemosphere. doi:10.1016/j.chemosphere.2014.12.018
Sura-de Jong M, Reynolds J, Richterova K, Musilova L, Staicu LC, Hrochova I, Cappa JJ, van der Lelie D, Frantik T, Sakmaryova I, Strejcek M, Cochran A, Lovecka P, Pilon-Smits EAH (2015) Selenium hyperaccumulators harbor a diverse endophytic bacterial community characterized by extreme selenium tolerance and growth promoting properties. Front Plant Sci (in revision)
Theisen J, Yee N (2014) The molecular basis for selenate reduction in Citrobacter freundii. Geomicrobiol J 31:875–883
Tomei FA, Barton LL, Lemanski CL, Zocco TG (1992) Reduction of selenate and selenite to elemental selenium by Wolinella succinogenes. Can J Microbiol 38:1328–1333
Tomei FA, Barton LL, Lemanski CL, Zocco TG, Fink NH, Sillerud LO (1995) Transformation of selenate and selenite to elemental selenium by Desulfovibrio desulfuricans. J Ind Microbiol 14:329–336
USEPA (2010) North San Francisco Bay selenium characterization study plan (2010–2012). http://www2.epagov/sites/production/files/documents/epa-r09-ow-2010-0976-0023-1pdf
van Hullebusch ED, Zandvoort MH, Lens PNL (2003) Metal immobilisation by biofilms: mechanisms and analytical tools. Rev Environ Sci Biotechnol 2:9–33
Wen H, Carignan J (2007) Reviews on atmospheric selenium: emissions speciation and fate. Atmos Environ 41:7151–7165
Yan W, Herzing AA, Kiely CJ, Zhang WX (2010) Nanoscale zero-valent iron (nZVI): aspects of the core-shell structure and reactions with inorganic species in water. J Contam Hydrol 118:96–104
Yudovich YE, Ketris MP (2006) Selenium in coal: a review. Int J Coal Geol 67:112–126
Zhang Y, Zahir ZA, Frankenberger WT Jr (2004) Fate of colloidal-particulate elemental selenium in aquatic systems. J Environ Qual 33:559–564
Zhang ES, Wang HL, Yan XX, Zhang LD (2005) Comparison of short-term toxicity between nano-Se and selenite in mice. Life Sci 76:1099–1109
Acknowledgments
We are grateful our collaborators Prof. Mehmet Oturan (Université Paris-Est, France) and Prof. Elizabeth Pilon-Smits (Colorado State University, USA) for useful discussions during the preparation of this manuscript. The authors would like to thank the European Commission for providing financial support through the Erasmus Mundus Joint Doctorate Programme ETeCoS3 (Environmental Technologies for Contaminated Solids, Soils and Sediments) under the grant agreement FPA n°2010-0009.
Author information
Authors and Affiliations
Corresponding author
Glossary
- Anaerobic granular sludge
-
Biomass in granular form produced by the auto-immobilization of mixed microbial populations and employed for the treatment of wastewater in up flow anaerobic granular sludge reactors
- Biogenic
-
Resulting from the action of a living organism; in the current context, Se nanoparticles produced by the metabolic activity of microorganisms.
- Chalcogen
-
Chemical element in group 16 of the periodic table; i.e., Oxygen (O), Sulfur (S), Selenium (Se), Tellurium (Te) and Polonium (Po). These elements are strongly associated with metal-bearing minerals.
- Chemogenic
-
Resulting from chemical action; in the current context, Se nanoparticles produced through the reduction of Se oxyanions using a chemical reducing agent.
- Colloidal system
-
System containing fine particles dispersed within a continuous medium that cannot be settled easily by gravitation.
Rights and permissions
About this article
Cite this article
Staicu, L.C., van Hullebusch, E.D. & Lens, P.N.L. Production, recovery and reuse of biogenic elemental selenium. Environ Chem Lett 13, 89–96 (2015). https://doi.org/10.1007/s10311-015-0492-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-015-0492-8