Abstract
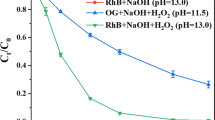
Rhodamine B can be degraded using Prussian blue as a photo-Fenton like reagent under λ > 420 nm visible irradiation. Kinetic studies show ln(C o/C t ) is linearly proportional to the reaction time during the photo-degradation process; thus, the degradation reaction obeys a pseudo-first order kinetic law. It is very interesting that the presence of salinity such as 0.1 M KCl can speed up greatly the degradation rate: the time to achieve 90.0% degradation ratio is shortened from 120.0 to 40.0 min under comparable conditions, which is very useful in the treatment of wastewaters with high content of salinity.





Similar content being viewed by others
References
Bacardit J, Stotzner J, Chamarro E, Esplugas S (2007) Effect of salinity on the photo-Fenton process. Ind Eng Chem Res 46:7615–7619
De Laat J, Truong G, Legube B (2004) A comparative study of the effect of chloride, sulfate, and nitrate ions on the rates of decomposition of H2O2 and organic compounds by Fe(II)/H2O2 and Fe(III)/H2O2. Chemosphere 55:715–723
Diagne M, Oturan N, Oturan M A, Sirés I (2008) UV-C light-enhanced photo-Fenton oxidation of methyl parathion. Environ Chem Lett. doi:10.1007/s10311-008-0162-1
Huston PL, Pignatello JJ (1996) Reduction of perchlorokanes by ferriox-generated carboxylate radical preceding mineralization by the photo-Fenton reaction. Environ Sci Technol 30:3457–3463
Itaya K, Uchida I (1986) Nature of intervalence charge-transfer bands in Prussian blues. Inorg Chem 25:389–394
Itaya K, Ataka T, Toshima S (1982) Spectroelectrochemistry and electrochemical preparation method of Prussian blue modified electrodes. J Am Chem Soc 104:4767–4772
Kiwi J, Lopez A, Nadtochenko V (2000) Mechanism and kinetics of the OH-radical intervention during Fenton oxidation in the presence of a significant amount of radical scavenger (Cl−). Environ Sci Technol 34:2162–2168
Lu M, Chang Y, Chen I, Huang Y (2005) Effect of chloride ions on the oxidation of aniline by Fenton’s reagent. J Environ Manag 75:177–182
Nadtochenko V, Kiwi J (1998) Photoinduced mineralization of xylidine by the Fenton reagent. 2. Implications of the precursors formed in the dark. Environ Sci Technol 32:3282–3285
Pignatello JJ, Oliveros E, Mackay A (2006) Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol 36:1–84
Pyrasch M, Tieke B (2001) Electro- and photoresponsive films of Prussian blue prepared upon multiple sequential adsorption. Langmuir 17:7706–7709
Sato O (2003) Optically switchable molecular solids: photoinduced spin-crossover, photochromism, and photoinduced magnetization. Acc Chem Res 36(9):692–700
Walling C, Goosen A (1973) Mechanism of ferric ion catalyzed decomposition of hydrogen peroxide. Effect of organic substrates. J Am Chem Soc 95:2987–2991
Zhao G, Feng J, Zhang Q, Li S, Chen H (2005) Synthesis and characterization of Prussian blue modified magnetite nanoparticles and its application to the electrocatalytic reduction of H2O2. Chem Mater 17:3154–3159
Acknowledgments
The authors gratefully acknowledge the financial supports by Ministry of Housing and Urban-Rural Development, China (Grant:06-K4-26); Provincial Key Laboratory of Environmental Science and Engineering, Jiangsu of China (Grant:2D051204).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, SQ., Cheng, S., Luo, L. et al. Degradation of dye rhodamine B under visible irradiation with Prussian blue as a photo-Fenton reagent. Environ Chem Lett 9, 31–35 (2011). https://doi.org/10.1007/s10311-009-0242-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-009-0242-x