Abstract
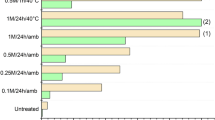
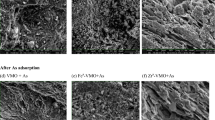
Adsorption of vanadate(V) from aqueous solution onto industrial solid ‘waste’ Fe(III)/Cr(III) hydroxide was investigated. HCl treated Fe(III)/Cr(III) hydroxide was found to be more efficient for the removal of vanadate(V) compared to untreated adsorbent. The adsorption follows second-order kinetics. Langmuir and Freundlich isotherms have been studied. The Langmuir adsorption capacity (Q 0) of the treated and untreated adsorbents was found to be 11.43 and 4.67 mg g−1, respectively. Thermodynamic parameters showed that the adsorption process was spontaneous and endothermic in the temperature range 32–60°C. Maximum adsorption was found at system pH 4.0. The adsorption mechanism was predominantly ion exchange. Effect of other anions such as phosphate, selenite, molybdate, nitrate, chloride, and sulfate on adsorption of vanadium has been examined.







Similar content being viewed by others
References
Alkan M, Demirbas O, Celikcapa S, Dogan M (2004) Sorption of acid red 57 from aqeuous solution onto sepiolite. J Hazard Mater B 116:135–145
Baes CF Jr, Mesmer RE (1976) Hydrolysis of cation. Wiley, New York
Blackmore DPT, Ellis J, Riley PJ (1996) Treatment of vanadium containing effluent by adsorption/coprecipitation with iron oxyhydroxide. Water Res 30:2512–2516
Dursun G, Cicek H, Dursun AY (2005) Adsorption of phenol from aqueous solution using carbonized beet pulp. J Hazard Mater B 125:175–182
Freundlich H (1906) Uber die adsorption in losungen. Z Phys Chem 57:387–470
Guibal E, Milot C, Tobin JM (1998) Metal-anion sorption by chitosan beads: equilibrium and kinetic studies. Ind Eng Chem Res 37:1454–1463
Guzman J, Saucedo J, Navarro R, Revilla J, Guibal E (2002) Vanadium interaction with chitosan: influence of polymer protection and metal speciation. Langmuir 18:1567–1573
Hayes KF, Papelis C, Leckie JO (1988) Modeling ionic strength effects on anion adsorption at hydrous oxide/solution interface. J Colloid Interface Sci 125:717–726
Hingston FJ, Posner AM, Quirk JP (1971) Competitive adsorption of negatively charged ligands on oxide surfaces. Discuss Faraday Soc 53:334
Jackson BP, Miller WP (2000) Effectiveness of phosphate and hydroxide for desorption of arsenic and selenium species from iron oxides. Soil Sci Soc Am J 64:1616–1622
Jansson-Charrier M, Guibal E, Delaghe B, Le Clorec P (1996) Vanadium (IV) sorption by chitosan: kinetics and equilibrium. Water Res 30:465–475
Kreller DI, Gibson G, van Loon GW, Horton JH (2002) Chemical force microscopy investigation of phosphate adsorption on the surface of Fe(III) oxyhydroxide articles. J Colloid Interface Sci 254:205–213
Kunz RG, Giannelli JF, Stensel HD (1976) Vanadium removal from industrial wastewaters. J WPCF 48:762–770
Lagergren S (1898) Zur throrie der sogenannten adsorption geloester stoffe. K Sven Vetenskapsakad Hadl 24:1–39
Langmuir I (1918) The adsorption of gases on plane surface of glass, mica and platinum. J Am Chem Soc 40:1361
Mckay G, Ho YS (1998) The adsorption of dyes from aqueous solution by peat. Chem Eng J 70:115–124
Naeem A, Westerhoff P, Mustafa S (2007) Vanadium removal by metal (hydr)oxide adsorbents. Water Res 41:1596–1602
Namasivayam C, Prathap K (2005) Recycling Fe(III)/Cr(III) hydroxide- and industrial solid waste for the removal of phophate from water. J Hazard Mater 123:127–134
Namasivayam C, Prathap K (2006) Removal of selenite using waste Fe(III)/Cr(III) hydroxide: Adsorption kinetics and isotherms. Toxicol Environ Chem 88:85–98
Namasivayam C, Prathap K (2007) Adsorptive removal of silica onto waste Fe(III)/Cr(III) hydroxide: kinetics and isotherms. Colloids Surf A Physicochem Eng Asp 295:55–60
Namasivayam C, Ranganathan K (1994) Recycling of waste Fe(III)/Cr(III) hydroxide for the removal of nickel from wastewater: adsorption and equilibrium studies. Waste Manage 14:709–716
Namasivayam C, Senthilkumar S (1998) Removal of arsenic(V) from aqueous solution using industrial solid waste: adsorption rates and equilibrium studies. Ind Eng Chem Res 37:4816–4822
Namasivayam C, Sumithra S (2004) Adsorptive removal of catechol on waste Fe(III)/Cr(III) hydroxide: equilibrium and kinetic study. Ind Eng Chem Res 43:7581–7587
Namasivayam C, Jayakumar R, Yamuna RT (1994) Dye removal from wastewater by adsorption on Fe(III)/Cr(III) hydroxide. Waste Manage 14:643–648
Nukatsuka I, Shimiza Y, Ohzeki K (2002) Determination of V(IV) and V(V) by electrothermal atomic absorption spectrometry following selective solid-phase extraction and the study on the change in the oxidation state of vanadium species in seawater during the sample storage. Anal Sci 18:1009–1014
Ortiz-Bernard I et al (2004) Vanadium respiration by Geobacter metallireducens: novel strategy for in situ removal of vanadium from groundwater. Appl Environ Microbiol 70:3091–3095
Ozacar M (2003) Equilibrium and kinetic modeling of adsorption of phosphorus on calcined alunite. Adsorption 9:125–132
Ozcan AS, Ozcan A (2004) Adsorption of acid dyes from aqueous solution onto acid activated-bentonite. J Colloid Interface Sci 276:39–46
Rayden JC, Syres KJ, Tilman RW (1987) Inorganic anion sorption and interaction with phosphate sorption by hydrous ferric oxide gel. J Soil Sci 38:211–217
Sontheimer H, Crittendren JC, Summers S (1988) Activated carbon for water treatment, chap. 3. Description of adsorption equilibria. DVGW Forschungstelle, Karlsruhe, Germany, p 112
Su C, Suarez DL (2000) Selenate and selenite sorption on iron oxides: an infrared and electrophoretic study. Soil Sci Soc Am J 64:101–111
Weng CH, Huang CP (2004) Adsorption characteristics of Zn(II) from dilute solution by fly ash. Colloids Surf A Physicochem Eng Asp 247:137–143
Zagulski I, Pawlowski L, Cichocki A (1980) Physicochemical methods for water and wastewater treatment. In: Pawlowski L (ed) Proceeding of the second international conference, Lublin 1979. Pergamon Press, Oxford, UK
Acknowledgments
The authors are grateful to Dr. P. Weidler, Institute for Technical Chemistry, Karlsruhe Research Center (Germany), for the analysis of surface area and pore size distribution of the adsorbent. Grateful acknowledgement is due to DAAD, Germany, for the Equipment Grant, which facilitated the experimental work. The authors are also grateful to the anonymous reviewers for the useful comments/suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prathap, K., Namasivayam, C. Adsorption of vanadate(V) on Fe(III)/Cr(III) hydroxide waste. Environ Chem Lett 8, 363–371 (2010). https://doi.org/10.1007/s10311-009-0234-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-009-0234-x