Abstract
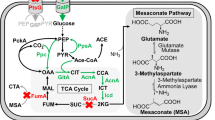
Glutaric acid is an important organic acid applied widely in different fields. Most previous researches have focused on the production of glutaric acid in various strains using the 5-aminovaleric acid (AMV) or pentenoic acid synthesis pathways. We previously utilized a five-step reversed adipic acid degradation pathway (RADP) in Escherichia coli BL21 (DE3) to construct strain Bgl146. Herein, we found that malonyl-CoA was strictly limited in this strain, and increasing its abundance could improve glutaric acid production. We, therefore, constructed a malonic acid uptake pathway in E. coli using matB (malonic acid synthetase) and matC (malonic acid carrier protein) from Clover rhizobia. The titer of glutaric acid was improved by 2.1-fold and 1.45-fold, respectively, reaching 0.56 g/L and 4.35 g/L in shake flask and batch fermentation following addition of malonic acid. Finally, the highest titer of glutaric acid was 6.3 g/L in fed-batch fermentation at optimized fermentation conditions.






Similar content being viewed by others
References
Adkins J (2013) Engineering Escherichia coli for renewable production of the 5-carbon polyamide building-blocks 5-aminovalerate and glutarate. Biotechnol Bioeng 110:1726–1734
Bermúdez M, León S, Alemán C, Muñoz-Guerra S (2000) Comparison of lamellar crystal structure and morphology of nylon 46 and nylon 5. Polymer 41:8961–8973
Castellan A, Bart JCJ, Cavallaro S (1991) Industrial production and use of adipic acid. Catal Today 9:237–254
Causey TB, Shanmugam KT, Yomano LP, Ingram LO (2004) Engineering Escherichia coli for efficient conversion of glucose to pyruvate. Proc Natl Acad Sci USA 101:2235–2240
Chen VC, Sadler G, Mccomb ME, Perreault H, Duckworth HW (2011) Characterization of specific binding by mass spectrometry: Associations of E. coli citrate synthase with NADH and 2-azidoATP. Int J Mass Spectrom 305:238–246
Deng Y, Mao Y (2015) Production of adipic acid by the native-occurring pathway in Thermobifida fusca B6. J Appl Microbiol 119(4):1057–1063
Fothergill JC, Guest JR (1977) Catabolism of L-Lysine by Pseudomonas aeruginosa. J Gen Microbiol 99:139–155
Heath RJ, Rock CO (1995) Regulation of malonyl-CoA metabolism by acyl-acyl carrier protein and β-ketoacyl-acyl carrier protein synthases in Escherichia coli. J Biol Chem 270:15531–15538
Jake A, Justin J, Nielsen DR (2013) Engineering Escherichia coli for renewable production of the 5-carbon polyamide building-blocks 5-aminovalerate and glutarate. Biotechnol Bioeng 110:1726–1734
Johnson AO, Gonzalez-Villanueva M, Wong L, Steinbüchel A, Tee KL, Xu P, Wong TS (2017) Design and application of genetically-encoded malonyl-CoA biosensors for metabolic engineering of microbial cell factories. Metab Eng 44:253–264
Johnson RW, Pollock CM, Cantrell RR (2005) Dicarboxylic acids. In: Van Nostrand's encyclopedia of chemistry. Wiley, New Jersey, p 789. 10.1002/0471740039.vec0808 2005
Xu JY, Xu Y, Xu Z, Zhai LH, Ye Y, Zhao Y, Chu XH, Tan MJ, Ye BC (2018) Protein acylation is a general regulatory mechanism in biosynthetic pathway of acyl-CoA-derived natural products. Cell Chem Biol 25:984–995
Kang SW, Hong SY, Ryoo HD, Rhyu GI, Yu-Sam K (1994) Kinetics of malonyl-CoA synthetase from Rhizobium trifolii and evidences for malonyl-AMP formation as a reaction intermediate. Bull Korean Chem Soc 15:327
Kim YS, Bang SK (1985) Malonyl coenzyme A synthetase. Purification and properties. J Biol Chem 260:5098
Kim YS, Chae HZ (1991) Purification and properties of malonyl-CoA synthetase from Rhizobium japonicum. Biochem J 273(Pt 3):511–516
Koebmann BJ, Westerhoff HV, Snoep JL, Dan N, Jensen PR (2002) The glycolytic flux in Escherichia coli is controlled by the demand for ATP. J Bacteriol 184:3909–3916
Krebs HA (1970) The history of the tricarboxylic acid cycle. Perspect Biol Med 14:154–172
Li SJ, Cronan JE (1992) The genes encoding the two carboxyltransferase subunits of Escherichia coli acetyl-coa carboxylase. J Biol Chem 267:16841–16847
Lussier FX, Colatriano D, Wiltshire Z, Page JE, Martin VJJ (2012) Engineering microbes for plant polyketide biosynthesis. Comput StructBiotechnol J 3:1–11
Magnuson K, Jackowski S, Rock CO, Cronan JE (1993) Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol Rev 57:522–542
Nazaret C, Heiske M, Thurley K, Mazat JP (2009) Mitochondrial energetic metabolism: a simplified model of TCA cycle with ATP production. J Theor Biol 258:455–464
Rohles CM, Gießelmann G, Kohlstedt M, Wittmann C, Becker J (2016) Systems metabolic engineering of Corynebacterium glutamicum for the production of the carbon-5 platform chemicals 5-aminovalerate and glutarate. Microb Cell Fact 15:154
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Shin JH, Park SH, Oh YH, Choi JW, Lee MH, Cho JS, Jeong KJ, Joo JC, Yu J, Si JP (2016) Metabolic engineering of Corynebacterium glutamicum for enhanced production of 5-aminovaleric acid. Microb Cell Fact 15:174
Si JP, Kim EY, Noh W, Park HM, Oh YH, Lee SH, Song BK, Jegal J, Sang YL (2013) Metabolic engineering of Escherichia coli for the production of 5-aminovalerate and glutarate as C5 platform chemicals. Metab Eng 16:42–47
Takamura Y, Nomura G (1988) Changes in the intracellular concentration of acetyl-CoA and malonyl-CoA in relation to the carbon and energy metabolism of Escherichia coli K12. J Gen Microbiol 134:2249–2253
Thomason MK, Storz G (2010) Bacterial antisense RNAs: how many are there, and what are they doing? Annu Rev Genet 44:167–188
Tokuyama K, Toya Y, Matsuda F, Cress BF, Koffas MA, Shimizu H (2019) Magnesium starvation improves production of malonyl-CoA-derived metabolites in Escherichia coli. Metab Eng 52:215–223
Wu J, Du G, Zhou J, Chen J (2013) Metabolic engineering of Escherichia coli for (2S)-pinocembrin production from glucose by a modular metabolic strategy. Metab Eng 16:48–55
Wu SF, Xu G, Ye G-Y (2015) Characterization of a tyramine receptor type 2 from hemocytes of rice stem borer, Chilo suppressalis. J Insect Physiol 75:39–46
Wu SG, Lian H, Wang Q, Tang YJ (2015) An ancient chinese wisdom for metabolic engineering: Yin-Yang. Microb Cell Fact 14:39
Xu P, Ranganathan S, Fowler ZL, Maranas CD, Koffas MAG (2011) Genome-scale metabolic network modeling results in minimal interventions that cooperatively force carbon flux towards malonyl-CoA. Metab Eng 13:578–587
Xue C, Zhang X, Zhirui YU, Zhao F, Wang M, Wenyu LU (2013) Up-regulated spinosad pathway coupling with the increased concentration of acetyl-CoA and malonyl-CoA contributed to the increase of spinosad in the presence of exogenous fatty acid. Biochem Eng J 81:47–53
Yu JL, Xia XX, Zhong JJ, Qian ZG (2017) Enhanced production of glutarate by using anaerobic-aerobic shift cultivation and an anaerobically inducible promoter in an engineered Escherichia coli. Process Biochem 62:S1359511317309625
Zha W, Rubin-Pitel SB, Shao Z, Zhao H (2009) Improving cellular malonyl-CoA level in Escherichia coli via metabolic engineering. Metab Eng 11:192–198
Zhang M, Chao G, Xiaoting G, Shiting G, Zhaoqi K, Dan X, Jinxin Y, Fei T, Wen Z, Wenyue D (2018) Increased glutarate production by blocking the glutaryl-CoA dehydrogenation pathway and a catabolic pathway involving l-2-hydroxyglutarate. Nat Commun 9:2114
Zhang M, Kang Z, Guo X, Guo S, Xiao D, Liu Y, Ma C, Xu P, Gao C (2019) Regulation of glutarate catabolism by GntR family regulator CsiR and LysR family regulator GcdR in Pseudomonas putida KT2440. Biology 10:e0157-01519. https://doi.org/10.1128/mBio.01570-19
Zhao M, Li GH, Deng Y (2018) Engineering Escherichia coli for glutarate production as the C-5 platform backbone. Appl Environ Microbiol. https://doi.org/10.1128/aem.00814-18
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFA0901400), the National Natural Science Foundation of China (21877053, 31600044), the Open Project Program of China–Canada Joint Lab of Food Nutrition and Health, Beijing Technology and Business University (BTBU), and the Fundamental Research Funds for the Central Universities (JUSRP51705A, JUSRP11964).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sui, X., Zhao, M., Liu, Y. et al. Enhancing glutaric acid production in Escherichia coli by uptake of malonic acid. J Ind Microbiol Biotechnol 47, 311–318 (2020). https://doi.org/10.1007/s10295-020-02268-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-020-02268-6