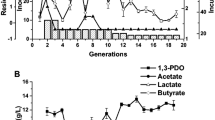
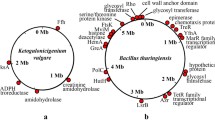
Abstract
Microbial consortia, with the merits of strong stability, robustness, and multi-function, played critical roles in human health, bioenergy, and food manufacture, etc. On the basis of ‘build a consortium to understand it’, a novel microbial consortium consisted of Gluconobacter oxydans, Ketogulonicigenium vulgare and Bacillus endophyticus was reconstructed to produce 2-keto-l-gulonic acid (2-KGA), the precursor of vitamin C. With this synthetic consortium, 73.7 g/L 2-KGA was obtained within 30 h, which is comparable to the conventional industrial method. A combined time-series proteomic and metabolomic analysis of the fermentation process was conducted to further investigate the cell–cell interaction. The results suggested that the existence of B. endophyticus and G. oxydans together promoted the growth of K. vulgare by supplying additional nutrients, and promoted the 2-KGA production by supplying more substrate. Meanwhile, the growth of B. endophyticus and G. oxydans was compromised from the competition of the nutrients by K. vulgare, enabling the efficient production of 2-KGA. This study provides valuable guidance for further study of synthetic microbial consortia.






Similar content being viewed by others
References
Brenner K, You L, Arnold FH (2008) Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol 26(9):483–489
Chen MT, Weiss R (2005) Artificial cell-cell communication in yeast Saccharomyces cerevisiae using signaling elements from Arabidopsis thaliana. Nat Biotechnol 23(12):1551–1555
Ding MZ, Song H, Wang EX, Liu Y, Yuan YJ (2016) Design and construction of synthetic microbial consortia in China. Synth Syst Biotechnol 1(4):230–235
Ding MZ, Zou Y, Song H, Yuan YJ (2014) Metabolomic analysis of cooperative adaptation between co-cultured Bacillus cereus and Ketogulonicigenium vulgare. Plos One 9(4):e94889
Dinh TN, Nagahisa K, Hirasawa T, Furusawa C, Shimizu AH (2008) Adaptation of Saccharomyces cerevisiae cells to high ethanol concentration and changes in fatty acid composition of membrane and cell size. Plos One 3(7):e2623
Du J, Bai W, Song H, Yuan YJ (2013) Combinational expression of sorbose/sorbosone dehydrogenases and cofactor pyrroloquinoline quinone increases 2-keto-l-gulonic acid production in Ketogulonicigenium vulgare–Bacillus cereus consortium. Metab Eng 19:50–56
Du J, Zhou J, Xue J, Song H, Yuan Y (2012) Metabolomic profiling elucidates community dynamics of the Ketogulonicigenium vulgare–Bacillus megaterium consortium. Metabolomics 8(5):960–973
Elowitz M, Lim WA (2010) Build life to understand it. Nature 468(7326):889
Gao Y, Yuan YJ (2011) Comprehensive quality evaluation of corn steep liquor in 2-keto-l-gulonic acid fermentation. J Agr Food Chem 59(18):9845–9853
Ghazali FM, Rahman RNZA, Salleh AB, Basri M (2004) Biodegradation of hydrocarbons in soil by microbial consortium. Int Biodeter Biodegr 54(1):61–67
Hays SG, Patrick WG, Ziesack M, Oxman N, Silver PA (2015) Better together: engineering and application of microbial symbioses. Curr Opin Biotech 36:40–49
Jia N, Ding MZ, Du J, Pan CH, Tian G, Lang JD, Fang JH, Gao F, Yuan YJ (2016) Insights into mutualism mechanism and versatile metabolism of Ketogulonicigenium vulgare Hbe602 based on comparative genomics and metabolomics studies. Sci Rep 6:23068
Jia N, Ding MZ, Zou Y, Gao F, Yuan YJ (2017) Comparative genomics and metabolomics analyses of the adaptation mechanism in Ketogulonicigenium vulgare-Bacillus thuringiensis consortium. Sci Rep 7:46759
Johns NI, Blazejewski T, Gomes ALC, Wang HH (2016) Principles for designing synthetic microbial communities. Curr Opin Microbio 31:146–153
Jones JA, Vernacchio VR, Collins SM, Shirke AN, Xiu Y, Englaender JA, Cress BF, McCutcheon CC, Linhardt RJ, Gross RA, Koffas MAG (2017) Complete biosynthesis of anthocyanins using E. coli polycultures. Mbio 8(3):e00621-17
Jones JA, Wang X (2017) Use of bacterial co-cultures for the efficient production of chemicals. Curr Opin Biotechnol 53:33–38
Klitgord N, Segrè D (2011) Ecosystems biology of microbial metabolism. Curr Opin Biotech 22(4):541–546
Lin T, Bai X, Hu Y, Li B, Yuan YJ, Song H, Yang Y, Wang J (2017) Synthetic Saccharomyces cerevisiae–Shewanella oneidensis consortium enables glucose-fed high-performance microbial fuel cell. AIChE J 63(6):1830–1838
Liu X, Li XB, Jiang J, Liu ZN, Qiao B, Li FF, Cheng JS, Sun X, Yuan YJ, Qiao J, Zhao GR (2018) Convergent engineering of syntrophic Escherichia coli, coculture for efficient production of glycosides. Metab Eng 47:243–253
Liu Y, Ding M, Ling W, Yang Y, Zhou X, Li BZ, Chen T, Nie Y, Wang M, Zeng B, Li X, Liu H, Sun B, Xu H, Zhang J, Jiao Y, Hou Y, Yang H, Xiao S, Lin Q, He X, Liao W, Jin Z, Xie Y, Zhang B, Li T, Lu X, Li J, Zhang F, Wu XL, Song H, Yuan YJ (2017) A three-species microbial consortium for power generation. Energ Environ Sci 10(7):1600–1609
Ma Q, Zhang W, Zhang L, Qiao B, Pan C, Yi H, Wang L, Yuan YJ (2012) Proteomic analysis of Ketogulonicigenium vulgare under glutathione reveals high demand for thiamin transport and antioxidant protection. Plos One 7(2):e32156
Ma Q, Zhou J, Zhang W, Meng X, Sun J, Yuan YJ (2011) Integrated proteomic and metabolomic analysis of an artificial microbial community for two-step production of vitamin C. Plos One 6(10):e26108
Ma Q, Zou Y, Lv Y, Song H, Yuan YJ (2014) Comparative proteomic analysis of experimental evolution of the Bacillus cereus-Ketogulonicigenium vulgare co-culture. Plos One 9(3):e91789
Maintinguer SI, Fernandes BS, Duarte ICS, Saavedra NK, Adorno MAT, Varesche MB (2008) Fermentative hydrogen production by microbial consortium. Int J Hydrogen Energ 33(16):4309–4317
Mannazzu I, Angelozzi D, Belviso S, Budroni M, Farris GA, Goffrini P, Lodi T, Marzona M, Bardi L (2008) Behaviour of Saccharomyces cerevisiae wine strains during adaptation to unfavourable conditions of fermentation on synthetic medium: cell lipid composition, membrane integrity, viability and fermentative activity. Int J Food Microbiol 21(1):84–91
Pan CH, Wang EX, Jia N, Jia N, Dong XT, Liu Y, Ding MZ, Yuan YJ (2017) Reconstruction of amino acid biosynthetic pathways increases the productivity of 2-keto-l-gulonic acid in Ketogulonicigenium vulgare-Bacillus endophyticus consortium via genes screening. J Ind Microbiol Biotechnol 44(7):1031–1040
Picataggio S (2009) Potential impact of synthetic biology on the development of microbial systems for the production of renewable fuels and chemicals. Curr Opin Biotech 20(3):325–329
Rahman KSM, Banat IM, Thahira J, Thayumanavan T, Lakshmanaperumalsamy P (2002) Bioremediation of gasoline contaminated soil by a bacterial consortium amended with poultry litter, coir pith and rhamnolipid biosurfactant. Bioresource Technol 81(1):25–32
Röling WFM, Ferrer M, Golyshin PN (2010) Systems approaches to microbial communities and their functioning. Curr Opin Biotech 21(4):532–538
Saeidi N, Wong CK, Lo TM, Nguyen HX, Ling H, Leong SSJ, Poh CL, Chang MW (2011) Engineering microbes to sense and eradicate Pseudomonas aeruginosa, a human pathogen. Mol Syst Biol 7(1):521
Shong J, Jimenez Diaz MR, Collins CH (2012) Towards synthetic microbial consortia for bioprocessing. Curr Opin Biotech 23(5):798–802
Shou W, Ram S, Vilar JMG (2007) Synthetic cooperation in engineered yeast populations. P Natl Acad Sci USA 104(6):1877–1882
Song H, Ding MZ, Jia XQ, Ma Q, Yuan YJ (2014) Synthetic microbial consortia from systematic analysis to construction and applications. Chem Soc Rev 43:6954–6981
Tkac J, Svitel J, Vostiar I, Navratil M, Gemeiner P (2009) Membrane-bound dehydrogenases from Gluconobacter sp.: Interfacial electrochemistry and direct bioelectrocatalysis. Bioelectrochemist 76(1):53–62
Wang EX, Ding MZ, Ma Q, Dong XT, Yuan YJ (2016) Reorganization of a synthetic microbial consortium for one-step vitamin C fermentation. Microb Cell Fact 15(1):21
Weckwerth W, Wenzel K, Fiehn O (2004) Process for the integrated extraction, identification and quantification of metabolites, proteins and RNA to reveal their co-regulation in biochemical networks. Proteomics 4(1):78–83
Wilmes P, Bowen BP, Thomas BC, Mueller RS, Denef VJ, VerBerkmoes NC, Hettich RL, Northen TR, Banfield JF (2010) Metabolome-proteome differentiation coupled to microbial divergence. MBio 1(5):728–736
You KM, Rosenfield CL, Knipple DC (2003) Ethanol tolerance in the yeast Saccharomyces cerevisiae is dependent on cellular oleic acid content. Appl Environ Microbiol 69(3):1499–1503
Zelezniak A, Andrejev S, Ponomarova O, Mende DR, Bork P, Patil KR (2015) Metabolic dependencies drive species co-occurrence in diverse microbial communities. Proc Natl Acad Sci USA 112(51):E7156
Zhang H, Pereira B, Li Z, Stephanopoulos G (2015) Engineering Escherichia coli coculture systems for the production of biochemical products. Proc Natl Acad Sci USA 112(27):8266–8271
Zhou J, Ma Q, Yi H, Wang L, Song H, Yuan YJ (2011) Metabolome profiling reveals metabolic cooperation between Bacillus megaterium and Ketogulonicigenium vulgare during induced swarm motility. Appl Environ Micro 77(19):7023–7030
Zhou K, Qiao K, Steven E, Gregory S (2015) Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat Biotechnol 33(4):377
Zhou X, Cahoon M, Rosa P, Hedstrom L (1997) Expression, purification, and characterization of inosine 5′-monophosphate dehydrogenase from Borrelia burgdorferi. J Biol Chem 272(35):21977–21981
Zuroff TR, Curtis WR (2012) Developing symbiotic consortia for lignocellulosic biofuel production. Appl Microbiol Biot 93(4):1423–1435
Acknowledgements
This work was funded by the Ministry of Science and Technology of China (“973″ Program: 2014CB745100), the National Natural Science Foundation of China (21676190, 21621004), Innovative Talents and Platform Program of Tianjin (16PTGCCX00140, 16PTSYJC00050).
Author information
Authors and Affiliations
Contributions
QM and YHB are co-first authors of this work; QM, YHB, MZD and YJY designed the project and drafted the manuscript; QM, YHB, EXW, BBZ, XTD, BQ, and MZD performed the experiments; QM, EXW and MZD did the omics analysis; MZD and YJY supervised the whole research and revised the manuscript. All authors read and approved the final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ma, Q., Bi, YH., Wang, EX. et al. Integrated proteomic and metabolomic analysis of a reconstructed three-species microbial consortium for one-step fermentation of 2-keto-l-gulonic acid, the precursor of vitamin C. J Ind Microbiol Biotechnol 46, 21–31 (2019). https://doi.org/10.1007/s10295-018-2096-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-2096-3