Abstract
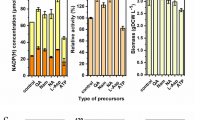
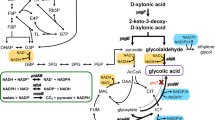
The NAD+/NADH ratio and the total NAD(H) play important roles for whole-cell biochemical redox transformations. After the carbon source is exhausted, the degradation of NAD(H) could contribute to a decline in the rate of a desired conversion. In this study, methods to slow the native rate of NAD(H) degradation were examined using whole-cell Escherichia coli with two model oxidative NAD+-dependent biotransformations. A high phosphate concentration (50 mM) was observed to slow NAD(H) degradation. We also constructed E. coli strains with deletions in genes coding several enzymes involved in NAD+ degradation. In shake-flask experiments, the total NAD(H) concentration positively correlated with conversion of xylitol to l-xylulose by xylitol 4-dehydrogenase, and the greatest conversion (80%) was observed using MG1655 nadR nudC mazG/pZE12-xdh/pCS27-nox. Controlled 1-L batch processes comparing E. coli nadR nudC mazG with a wild-type background strain demonstrated a 30% increase in final l-xylulose concentration (5.6 vs. 7.9 g/L) and a 25% increase in conversion (0.53 vs. 0.66 g/g). MG1655 nadR nudC mazG was also examined for the conversion of galactitol to l-tagatose by galactitol 2-dehydrogenase. A batch process using 15 g/L glycerol and 10 g/L galactitol generated over 9.4 g/L l-tagatose, corresponding to 90% conversion and a yield of 0.95 g l-tagatose/g galactitol consumed. The results demonstrate the value of minimizing NAD(H) degradation as a means to improve NAD+-dependent biotransformations.












Similar content being viewed by others
References
Aarnikunnas JS, Pihlajaniemi A, Palva A, Leisola M, Nyyssola A (2006) Cloning and expression of a xylitol-4-dehydrogenase gene from Pantoea ananatis. Appl Environ Microbiol 72(1):368–377
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:1
Baek SH, Park SJ, Lee HG (2010) d-Psicose, a sweet monosaccharide, ameliorate hyperglycemia, and dyslipidemia in C57BL/6 J db/db Mice. J Food Sci 75(2):H49–H53
Bao T, Zhang X, Rao Z, Zhao X, Zhang R, Yang T, Xu Z, Yang S (2014) Efficient whole-cell biocatalyst for acetoin production with NAD+ regeneration system through homologous co-expression of 2, 3-butanediol dehydrogenase and NADH oxidase in engineered Bacillus subtilis. PLoS ONE 9(7):e102951
Battley EH (1991) Calculation of the heat of growth of Escherichia coli K-12 cells on succinic acid. Biotechnol Bioeng 37(4):334–343
Battley EH (2003) Absorbed heat and heat of formation of dried microbial biomass. J Therm Anal Calorim 74(3):709–721
Bernofsky C, Swan M (1973) An improved cycling assay for nicotinamide adenine dinucleotide. Anal Biochem 53(2):452–458
Berrı́os-Rivera SJ, KY San, GN Bennett (2002) The effect of NAPRTase overexpression on the total levels of NAD, the NADH/NAD+ ratio, and the distribution of metabolites in Escherichia coli. Metab Eng 4(3):238–247
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci 97(12):6640–6645
Eiteman MA, Chastain MJ (1997) Optimization of the ion-exchange analysis of organic acids from fermentation. Anal Chim Acta 228(1–2):69–75
Foster JW, Park YK, Penfound T, Fenger T, Spector MP (1990) Regulation of NAD metabolism in Salmonella typhimurium: molecular sequence analysis of the bifunctional nadR regulator and the nadA-pnuC operon. J Bacteriol 172(8):4187–4196
Frick DN, Bessman MJ (1995) Cloning, purification, and properties of a novel NADH pyrophosphatase evidence for a nucleotide pyrophosphatase catalytic domain in MutT-like enzymes. J Biol Chem 270(4):1529–1534
Granström TB, Takata G, Tokuda M, Izumori K (2004) Izumoring: a novel and complete strategy for bioproduction of rare sugars. J Biosci Bioeng 97(2):89–94
Goodwin TE, Cousins KR, Crane HM, Eason PO, Freyaldenhoven TE, Harmon CC, King BK, LaRocca CD, Lile RL, Orlicek SG (1998) Synthesis of two new maytansinoid model compounds from carbohydrate precursors. J Carbo Chem 17(3):323–339
Gumina G, Song GY, Chu CK (2001) l-Nucleosides as chemotherapeutic agents. FEMS Microbiol Lett 202(1):9–15
Han Q, Eiteman MA (2017) Coupling xylitol dehydrogenase with NADH oxidase improves l-xylulose production in Escherichia coli culture. Enzyme Microb Technol 106:106–113
Heuser F, Schroer K, Lütz S, Bringer-Meyer S, Sahm H (2007) Enhancement of the NAD (P)(H) pool in Escherichia coli for biotransformation. Eng Life Sci 7(4):343–353
Heuser F, Marin K, Kaup B, Bringer S, Sahm H (2009) Improving d-mannitol productivity of Escherichia coli: impact of NAD, CO2 and expression of a putative sugar permease from Leuconostoc pseudomesenteroides. Metab Eng 11(3):178–183
Heyland J, Blank LM, Schmid A (2011) Quantification of metabolic limitations during recombinant protein production in Escherichia coli. J Biotechnol 155:178–184
Huwig A, Emmel S, Jäkel G, Giffhorn F (1997) Enzymatic synthesis of l-tagatose from galactitol with galactitol dehydrogenase from Rhodobacter sphaeroides D. Carbohydr Res 305(3–4):337–339
Imsande J, Pardee AB (1962) Regulation of pyridine nucleotide biosynthesis in Escherichia coli. J Biol Chem 237(4):1305–1308
Jeong LS, Schinazi RF, Beach JW, Kim HO, Nampalli S, Shanmuganathan K, Alves AJ, McMillan A, Chu CK, Mathis R (1993) Asymmetric synthesis and biological evaluation of. beta.-l-(2R, 5S)-and. alpha.-l-(2R, 5R)-1, 3-oxathiolane-pyrimidine and-purine nucleosides as potential anti-HIV agents. J Med Chem 36(2):181–195
Kakehi K, Usuda Y, Tabira Y, Sugimoto S (2007) Complete deficiency of 5′-nucleotidase activity in Escherichia coli leads to loss of growth on purine nucleotides but not of their excretion. J Mol Microbiol Biotechnol 13:96–104
Khan AR, Tokunaga H, Yoshida K, Izumori K (1991) Conversion of xylitol to l-xylulose by Alcaligenes sp. 701B-cells. J Ferment Bioeng 72(6):488–490
Kuroda A, Nomura K, Ohtomo R, Kato J, Ikeda T, Takiguchi N, Ohtake H, Kornberg A (2001) Role of inorganic polyphosphate in promoting ribosomal protein degradation by the Lon protease in E. coli. Science 293(5530):705–708
Ma T, Lin JS, Newton MG, Cheng YC, Chu CK (1997) Synthesis and anti-hepatitis B virus activity of 9-(2-deoxy-2-fluoro-beta-l-arabinofuranosyl) purine nucleosides. J Med Chem 40(17):2750–2754
Ma J, Gou D, Liang L, Liu R, Chen X, Zhang C, Zhang J, Chen K, Jiang M (2013) Enhancement of succinate production by metabolically engineered Escherichia coli with co-expression of nicotinic acid phosphoribosyltransferase and pyruvate carboxylase. Appl Microbiol Biotechnol 97(15):6739–6747
Mathé C, Gosselin G (2006) l-Nucleoside enantiomers as antivirals drugs: a mini-review. Antivir Res 71(2):276–281
Moran EJ, Tellew JE, Zhao Z, Armstrong RW (1993) Dehydroamino acid derivatives from d-arabinose and l-serine: synthesis of models for the azinomycin antitumor antibiotics. J Org Chem 58(27):7848–7859
Nobelmann B, Lengeler JW (1996) Molecular analysis of the gat genes from Escherichia coli and of their roles in galactitol transport and metabolism. J Bacteriol 178(23):6790–6795
O’Handley SF, Frick DN, Dunn CA, Bessman MJ (1998) Orf186 represents a new member of the Nudix hydrolases, active on adenosine (5′) triphospho (5′) adenosine, ADP-ribose, and NADH. J Biol Chem 273(6):3192–3197
Poonperm W, Takata G, Morimoto K, Granström TB, Izumori K (2007) Production of l-xylulose from xylitol by a newly isolated strain of Bacillus pallidus Y25 and characterization of its relevant enzyme xylitol dehydrogenase. Enzyme Microb Technol 40(5):1206–1212
San KY, Bennett GN, Berrı́os-Rivera SJ, Vadali RV, Yang YT, Horton E, Rudolph FB, Sariyar B, Blackwood K (2002) Metabolic engineering through cofactor manipulation and its effects on metabolic flux redistribution in Escherichia coli. Metab Eng 4(2):182–192
Sánchez AM, Bennett GN, San KY (2005) Effect of different levels of NADH availability on metabolic fluxes of Escherichia coli chemostat cultures in defined medium. J Biotechnol 117(4):395–405
Schurig-Briccio LA, Rintoul MR, Volentini SI, Farías RN, Baldomà L, Badía J, Rodríguez-Montelongo L, Rapisarda VA (2008) A critical phosphate concentration in the stationary phase maintains ndh gene expression and aerobic respiratory chain activity in Escherichia coli. FEMS Microbiol Lett 284(1):76–83
Schurig-Briccio LA, Farías RN, Rintoul MR, Rapisarda VA (2009) Phosphate-enhanced stationary-phase fitness of Escherichia coli is related to inorganic polyphosphate level. J Bacteriol 191(13):4478–4481
Schurig-Briccio LA, Farías RN, Rodríguez-Montelongo L, Rintoul MR, Rapisarda VA (2009) Protection against oxidative stress in Escherichia coli stationary phase by a phosphate concentration-dependent genes expression. Arch Biochem Biophys 483:106–110
Vemuri GN, Eiteman MA, Altman E (2006) Increased recombinant protein production in Escherichia coli strains with overexpressed water-forming NADH oxidase and a deleted ArcA regulatory protein. Biotechnol Bioeng 94(3):538–542
Vemuri GN, Altman E, Sangurdekar DP, Khodursky AB, Eiteman MA (2006) Overflow metabolism in Escherichia coli during steady-state growth: transcriptional regulation and effect of the redocx ratio. Appl Environ Microbiol 72(5):3653–3661
Wang L, Zhou YJ, Ji D, Lin X, Liu Y, Zhang Y, Liu W, Zhao ZK (2014) Identification of UshA as a major enzyme for NAD degradation in Escherichia coli. Enzyme Microb Technol 58:75–79
Wubbolts MG, Terpstra P, van Beilen JB, J Kingma, Meesters HA, Witholt B (1990) Variation of cofactor levels in Escherichia coli. Sequence analysis and expression of the pncB gene encoding nicotinic acid phosphoribosyltransferase. J Biol Chem 265(29):17665–17672
Xiao Z, Lv C, Gao C, Qin J, Ma C, Liu Z, Liu P, Li L, Xu P (2010) A novel whole-cell biocatalyst with NAD+ regeneration for production of chiral chemicals. PLoS ONE 5(1):e8860
Zhang J, Inouye M (2002) MazG, a nucleoside triphosphate pyrophosphohydrolase, interacts with Era, an essential GTPase in Escherichia coli. J Bacteriol 184(19):5323–5329
Zhou YJ, Yang W, Wang L, Zhu Z, Zhang S, Zhao ZK (2013) Engineering NAD+ availability for Escherichia coli whole-cell biocatalysis: a case study for dihydroxyacetone production. Microb Cell Fact 12(1):103
Acknowledgements
The authors thank Sarah Lee for the technical assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Han, Q., Eiteman, M.A. Enhancement of NAD(H) pool for formation of oxidized biochemicals in Escherichia coli. J Ind Microbiol Biotechnol 45, 939–950 (2018). https://doi.org/10.1007/s10295-018-2072-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-2072-y