Abstract
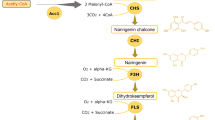
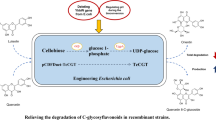
Kaempferol and astragalin are used as standards to assess the quality of Ginkgo biloba extract and Radix astragali, respectively, and possess numerous biological properties. In this study, we constructed a recombinant strain with a highly efficient biosynthetic pathway of kaempferol by screening key enzyme genes, designing a synthetic fusion enzyme and increasing the gene copy number. By optimizing conversion and fed-batch fermentation conditions, maximal kaempferol production reached 1184.2 ± 16.5 mg/L, which represents the highest yield of kaempferol from naringenin reported to date. Based on this result, glycosyltransferase (AtUGT78D2) and an efficient UDP-glucose synthesis pathway were introduced into the recombinant strain to produce astragalin, resulting in maximal astragalin production at 1738.5 ± 24.8 mg/L without kaempferol accumulation. The efficient synthesis pathway described in this study for kaempferol and astragalin biosynthesis can be widely used for flavonoid biosynthesis in Escherichia coli.











Similar content being viewed by others
References
Brazier-Hicks M, Edwards R (2013) Metabolic engineering of the flavone-C-glycoside pathway using polyprotein technology. Metab Eng 16:11–20. https://doi.org/10.1016/j.ymben.2012.11.004
Britsch L, Grisebach H (1986) Purification and characterization of (2S)-flavanone 3-hydroxylase from Petunia hybrida. Eur J Biochem 156(3):569–577. https://doi.org/10.1111/j.1432-1033.1986.tb09616.x
Brunner G (2005) Supercritical fluids: technology and application to food processing. J Food Eng 67(1):21–33. https://doi.org/10.1016/j.jfoodeng.2004.05.060
Chemler JA, Lock LT, Koffas MA, Tzanakakis ES (2007) Standardized biosynthesis of flavan-3-ols with effects on pancreatic beta-cell insulin secretion. Appl Microbiol Biotechnol 77(4):797–807. https://doi.org/10.1007/s00253-007-1227-y
Chen AY, Chen YC (2013) A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem 138(4):2099–2107. https://doi.org/10.1016/j.foodchem.2012.11.139
Chen Z, Sun X, Li Y, Yan Y, Yuan Q (2017) Metabolic engineering of Escherichia coli for microbial synthesis of monolignols. Metab Eng 39:102–109. https://doi.org/10.1016/j.ymben.2016.10.021
Choi J, Kang HJ, Kim SZ, Kwon TO, Jeong SI, Jang SI (2013) Antioxidant effect of astragalin isolated from the leaves of Morus alba L. against free radical-induced oxidative hemolysis of human red blood cells. Arch Pharm Res 36(7):912–917. https://doi.org/10.1007/s12272-013-0090-x
De Bruyn F, Van Brempt M, Maertens J, Van Bellegem W, Duchi D, De Mey M (2015) Metabolic engineering of Escherichia coli into a versatile glycosylation platform: production of bio-active quercetin glycosides. Microb Cell Fact 14:138. https://doi.org/10.1186/s12934-015-0326-1
Duan L, Ding W, Liu X, Cheng X, Cai J, Hua E, Jiang H (2017) Biosynthesis and engineering of kaempferol in Saccharomyces cerevisiae. Microb Cell Fact 16(1):165. https://doi.org/10.1186/s12934-017-0774-x
Eichenberger M, Lehka BJ, Folly C, Fischer D, Martens S, Simon E, Naesby M (2017) Metabolic engineering of Saccharomyces cerevisiae for de novo production of dihydrochalcones with known antioxidant, antidiabetic, and sweet tasting properties. Metab Eng 39:80–89. https://doi.org/10.1016/j.ymben.2016.10.019
Fowler ZL, Gikandi WW, Koffas MA (2009) Increased malonyl coenzyme A biosynthesis by tuning the Escherichia coli metabolic network and its application to flavanone production. Appl Environ Microbiol 75(18):5831–5839. https://doi.org/10.1128/aem.00270-09
Huber R, Scheidle M, Dittrich B, Klee D, Buchs J (2009) Equalizing growth in high-throughput small scale cultivations via precultures operated in fed-batch mode. Biotechnol Bioeng 103(6):1095–1102. https://doi.org/10.1002/bit.22349
Hwang EI, Kaneko M, Ohnishi Y, Horinouchi S (2003) Production of plant-specific flavanones by Escherichia coli containing an artificial gene cluster. Appl Environ Microbiol 69(5):2699–2706. https://doi.org/10.1128/AEM.69.5.2699-2706.2003
Jang S, Xiu Y, Kang TJ, Lee SH, Koffas MAG, Jung GY (2017) Development of artificial riboswitches for monitoring of naringenin in vivo. ACS Synth Biol 6:2077–2085. https://doi.org/10.1021/acssynbio.7b00128
Jones JA, Vernacchio VR, Sinkoe AL, Collins SM, Ibrahim MHA, Lachance DM, Hahn J, Koffas MAG (2016) Experimental and computational optimization of an Escherichia coli co-culture for the efficient production of flavonoids. Metab Eng 35:55–63. https://doi.org/10.1016/j.ymben.2016.01.006
Ke M, Hu XQ, Ouyang J, Dai B, Xu Y (2012) The effect of astragalin on the VEGF production of cultured Muller cells under high glucose conditions. Biomed Mater Eng 22(1–3):113–119. https://doi.org/10.3233/bme-2012-0696
Kim MS, Kim SH (2011) Inhibitory effect of astragalin on expression of lipopolysaccharide-induced inflammatory mediators through NF-kappaB in macrophages. Arch Pharm Res 34(12):2101–2107. https://doi.org/10.1007/s12272-011-1213-x
Koopman F, Beekwilder J, Crimi B, van Houwelingen A, Hall RD, Bosch D, van Maris AJ, Pronk JT, Daran JM (2012) De novo production of the flavonoid naringenin in engineered Saccharomyces cerevisiae. Microb Cell Fact 11:155. https://doi.org/10.1186/1475-2859-11-155
Kwon HJ, Park YD (2012) Determination of astragalin and astragaloside content in Radix Astragali using high-performance liquid chromatography coupled with pulsed amperometric detection. J Chromatogr A 1232:212–217. https://doi.org/10.1016/j.chroma.2011.12.035
Leonard E, Lim KH, Saw PN, Koffas MA (2007) Engineering central metabolic pathways for high-level flavonoid production in Escherichia coli. Appl Environ Microbiol 73(12):3877–3886. https://doi.org/10.1128/aem.00200-07
Li C, Bai Y, Li S, Chen H, Han X, Zhao H, Shao J, Park SU, Wu Q (2012) Cloning, characterization, and activity analysis of a flavonol synthase gene FtFLS1 and its association with flavonoid content in tartary buckwheat. J Agric Food Chem 60(20):5161–5168. https://doi.org/10.1021/jf205192q
Li F, Wang W, Cao Y, Liang D, Zhang W, Zhang Z, Jiang H, Guo M, Zhang N (2014) Inhibitory effects of astragalin on lipopolysaccharide-induced inflammatory response in mouse mammary epithelial cells. J Surg Res 192(2):573–581. https://doi.org/10.1016/j.jss.2014.05.059
Lin GZ, Lian YJ, Ryu JH, Sung MK, Park JS, Park HJ, Park BK, Shin JS, Lee MS, Cheon CI (2007) Expression and purification of His-tagged flavonol synthase of Camellia sinensis from Escherichia coli. Protein Expr Purif 55(2):287–292. https://doi.org/10.1016/j.pep.2007.05.013
Lukacin R, Wellmann F, Britsch L, Martens S, Matern U (2003) Flavonol synthase from Citrus unshiu is a bifunctional dioxygenase. Phytochemistry 62(3):287–292. https://doi.org/10.1016/S0031-9422(02)00567-8
Malla S, Pandey RP, Kim BG, Sohng JK (2013) Regiospecific modifications of naringenin for astragalin production in Escherichia coli. Biotechnol Bioeng 110(9):2525–2535. https://doi.org/10.1002/bit.24919
Miyahisa I, Kaneko M, Funa N, Kawasaki H, Kojima H, Ohnishi Y, Horinouchi S (2005) Efficient production of (2S)-flavanones by Escherichia coli containing an artificial biosynthetic gene cluster. Appl Microbiol Biotechnol 68(4):498–504. https://doi.org/10.1007/s00253-005-1916-3
Owens DK, Crosby KC, Runac J, Howard BA, Winkel BS (2008) Biochemical and genetic characterization of Arabidopsis flavanone 3β-hydroxylase. Plant Physiol Biochem 46(10):833–843. https://doi.org/10.1016/j.plaphy.2008.06.004
Pei J, Dong P, Wu T, Zhao L, Cao F, Tang F (2017) Characterization flavanone 3β-hydroxylase expressed from Populus euphratica in Escherichia coli and its application in dihydroflavonol production. Appl Biochem Microbiol 53(3):318–324. https://doi.org/10.1134/S0003683817030127
Pei J, Dong P, Wu T, Zhao L, Fang X, Cao F, Tang F, Yue Y (2016) Metabolic engineering of Escherichia coli for astragalin biosynthesis. J Agric Food Chem 64(42):7966–7972. https://doi.org/10.1021/acs.jafc.6b03447
Priscila G, Fernandez FJ, Absalon AE, Suarez Mdel R, Sainoz M, Barrios-Gonzalez J, Mejia A (2008) Expression of the bacterial hemoglobin gene from Vitreoscilla stercoraria increases rifamycin B production in Amycolatopsis mediterranei. J Biosci Bioeng 106:493–497. https://doi.org/10.1263/jbb.106.493
Rajendran P, Rengarajan T, Nandakumar N, Palaniswami R, Nishigaki Y, Nishigaki I (2014) Kaempferol, a potential cytostatic and cure for inflammatory disorders. Eur J Med Chem 86:103–112. https://doi.org/10.1016/j.ejmech.2014.08.011
Rodriguez A, Strucko T, Stahlhut SG, Kristensen M, Svenssen DK, Forster J, Nielsen J, Borodina I (2017) Metabolic engineering of yeast for fermentative production of flavonoids. Bioresour Technol 245:1645–1654. https://doi.org/10.1016/j.biortech.2017.06.043
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. Cold Spring Harbor Laboratory Press, New York
Shen G, Pang Y, Wu W, Deng Z, Zhao L, Cao Y, Sun X, Tang K (2006) Cloning and characterization of a flavanone 3-hydroxylase gene from Ginkgo biloba. Biosci Rep 26(1):19–29. https://doi.org/10.1007/s10540-006-9007-y
Shi X, Xie J, Liao S, Wu T, Zhao LG, Ding G, Wang Z, Xiao W (2017) High-level expression of recombinant thermostable beta-glucosidase in Escherichia coli by regulating acetic acid. Bioresour Technol 241:795–801. https://doi.org/10.1016/j.biortech.2017.05.105
Stahlhut SG, Siedler S, Malla S, Harrison SJ, Maury J, Neves AR, Forster J (2015) Assembly of a novel biosynthetic pathway for production of the plant flavonoid fisetin in Escherichia coli. Metab Eng 31:84–93. https://doi.org/10.1016/j.ymben.2015.07.002
Trantas EA, Koffas MA, Xu P, Ververidis F (2015) When plants produce not enough or at all: metabolic engineering of flavonoids in microbial hosts. Front Plant Sci 6:7. https://doi.org/10.3389/fpls.2015.00007
Wei Y, Xie Q, Fisher D, Sutherland IA (2011) Separation of patuletin-3-O-glucoside, astragalin, quercetin, kaempferol and isorhamnetin from Flaveria bidentis (L.) Kuntze by elution-pump-out high-performance counter-current chromatography. J Chromatogr A 1218(36):6206–6211. https://doi.org/10.1016/j.chroma.2011.01.058
Wu J, Yu O, Du G, Zhou J, Chen J (2014) Fine-tuning of the fatty acid pathway by synthetic antisense RNA for enhanced (2S)-naringenin production from l-Tyrosine in Escherichia coli. Appl Environ Microbiol 80(23):7283–7292. https://doi.org/10.1128/aem.02411-14
Xiong D, Lu S, Wu J, Liang C, Wang W, Jin JM, Tang SY (2017) Improving key enzyme activity in phenylpropanoid pathway with a designed biosensor. Metab Eng 40:115–123. https://doi.org/10.1016/j.ymben.2017.01.006
Xu P, Ranganathan S, Fowler ZL, Maranas CD, Koffas MA (2011) Genome-scale metabolic network modeling results in minimal interventions that cooperatively force carbon flux towards malonyl-CoA. Metab Eng 13(5):578–587. https://doi.org/10.1016/j.ymben.2011.06.008
Xue YL, Miyakawa T, Hayashi Y, Okamoto K, Hu F, Mitani N, Furihata K, Sawano Y, Tanokura M (2011) Isolation and tyrosinase inhibitory effects of polyphenols from the leaves of persimmon, Diospyros kaki. J Agric Food Chem 59(11):6011–6017. https://doi.org/10.1021/jf200940h
Yu K, Liu C, Kim BG, Lee DY (2015) Synthetic fusion protein design and applications. Biotechnol Adv 33(1):155–164. https://doi.org/10.1016/j.biotechadv.2014.11.005
Zhao S, Jones JA, Lachance DM, Bhan N, Khalidi O, Venkataraman S, Wang Z, Koffas MA (2015) Improvement of catechin production in Escherichia coli through combinatorial metabolic engineering. Metab Eng 28:43–53. https://doi.org/10.1016/j.ymben.2014.12.002
Zhou YJ, Gao W, Rong Q, Jin G, Chu H, Liu W, Yang W, Zhu Z, Li G, Zhu G, Huang L, Zhao ZK (2012) Modular pathway engineering of diterpenoid synthases and the mevalonic acid pathway for miltiradiene production. J Am Chem Soc 134(6):3234–3241. https://doi.org/10.1021/ja2114486
Acknowledgements
This work was supported by the National Key R&D Program of China (2017YFD0600805), the National Natural Science Foundation of China (31570565), the Open Foundation of Jiangsu Provincial Engineering Laboratory for Biomass Conversion and Process Integration (JPELBCPI2017002), the Qing Lan Project and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pei, J., Chen, A., Dong, P. et al. Modulating heterologous pathways and optimizing fermentation conditions for biosynthesis of kaempferol and astragalin from naringenin in Escherichia coli. J Ind Microbiol Biotechnol 46, 171–186 (2019). https://doi.org/10.1007/s10295-018-02134-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-02134-6