Abstract
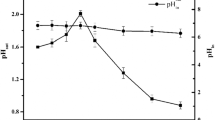
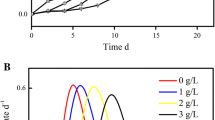
Acidophilic microorganisms involved in uranium bioleaching are usually suppressed by dissolved fluoride ions, eventually leading to reduced leaching efficiency. However, little is known about the regulation mechanisms of microbial resistance to fluoride. In this study, the resistance of Acidithiobacillus ferrooxidans ATCC 23270 to fluoride was investigated by detecting bacterial growth fluctuations and ferrous or sulfur oxidation. To explore the regulation mechanism, a whole genome microarray was used to profile the genome-wide expression. The fluoride tolerance of A. ferrooxidans cultured in the presence of FeSO4 was better than that cultured with the S0 substrate. The differentially expressed gene categories closely related to fluoride tolerance included those involved in energy metabolism, cellular processes, protein synthesis, transport, the cell envelope, and binding proteins. This study highlights that the cellular ferrous oxidation ability was enhanced at the lower fluoride concentrations. An overview of the cellular regulation mechanisms of extremophiles to fluoride resistance is discussed.







Similar content being viewed by others
References
Acosta M, Beard S, Ponce JVG, Vera M, Mobarec JC, Jerez CA (2005) Identification of putative sulfurtransferase genes in the extremophilic Acidithiobacillus ferrooxidans ATCC 23270 genome: structural and functional characterization of the proteins. OMICS 9:13
Alvarez S, Jerez CA (2004) Copper ions stimulate polyphosphate degradation and phosphate efflux in Acidithiobacillus ferrooxidans. Appl Environ Microbiol 70:5177–5182
Bhatti TM, Antti V, Martti L, Tuovinen OH (1998) Dissolution of uraninite in acid solutions. J Chem Technol Biotechnol 73:259–263
Borole AP, Hamilton CY (2011) Using acidithiobacillus, leptospirillium and/or sulfolobus as bioreactor for removing mercury from fossil fuels; bioremediation; pollution control; biodegradation; biooxidation: US 7998724 B2
Brierley J, Kuhn M (2010) Fluoride toxicity in a chalcocite bioleach heap process. Hydrometallurgy 104:410–413
Brigham CJ, Speth DR, Rha C, Sinskey AJ (2012) Whole-genome microarray and gene deletion studies reveal regulation of the polyhydroxyalkanoate production cycle by the stringent response in Ralstonia eutropha H16. Appl Environ Microbiol 78:8033–8044
Chakraborty S, Mukherjee A, Khuda-Bukhsh AR, Das TK (2014) Cadmium-induced oxidative stress tolerance in cadmium resistant Aspergillus foetidus: its possible role in cadmium bioremediation. Ecotoxicol Environ Saf 106:46–53
Dopson M, Halinen AK, Rahunen N, Boström D, Sundkvist JE, Riekkola-Vanhanen M, Kaksonen AH, Puhakka JA (2008) Silicate mineral dissolution during heap bioleaching. Biotechnol Bioeng 99:811–820
Dopson M, Holmes DS (2014) Metal resistance in acidophilic microorganisms and its significance for biotechnologies. Appl Microbiol Biotechnol 98:8133–8144
Fisher MA, Plikaytis BB, Shinnick TM (2002) Microarray analysis of the Mycobacterium tuberculosis transcriptional response to the acidic conditions found in phagosomes. J Bacteriol 184:4025–4032
Gao H, Wang Y, Liu X, Yan T, Wu L, Alm E, Arkin A, Thompson DK, Zhou J (2004) Global transcriptome analysis of the heat shock response of Shewanella oneidensis. J Bacteriol 186:7796–7803
Gao H, Yang ZK, Wu L, Thompson DK, Zhou J (2006) Global transcriptome analysis of the cold shock response of Shewanella oneidensis MR-1 and mutational analysis of its classical cold shock proteins. J Bacteriol 188:4560–4569
Gehrke T, Hallmann R, Kinzler K, Sand W (2001) The EPS of Acidithiobacillus ferrooxidans—a model for structure-function relationships of attached bacteria and their physiology. Water Sci Technol 43:159–167
Gholami RM, Borghei SM, Mousavi SM (2011) Bacterial leaching of a spent Mo–Co–Ni refinery catalyst using Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. Hydrometallurgy 106:26–31
Gutknecht J, Walter A (1981) Hydrofluoric and nitric acid transport through lipid bilayer membranes. BBA Bioenergetics 644:153–156
Helmann JD, Wu MFW, Kobel PA, Gamo F-J, Wilson M, Morshedi MM, Navre M, Paddon C (2001) Global transcriptional response of Bacillus subtilis to heat shock. J Bacteriol 183:7318–7328
Jin D, Kong X, Li Y, Bai Z, Zhuang G, Zhuang X, Deng Y (2015) Biodegradation of di-n-butyl phthalate by Achromobacter sp. isolated from rural domestic wastewater. Int J Env Res Public Health 12:13510–13522
Kates M (1996) Structural analysis of phospholipids and glycolipids in extremely halophilic archaebacteria. J Microbiol Methods 25:113–128. doi:10.1016/0167-7012(96)00010-3
Ko M-S, Park H-S, Kim K-W, Lee J-U (2013) The role of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans in arsenic bioleaching from soil. Environ Geochem Health 35:727–733
Kruger MC, Bertin PN, Heipieper HJ, Arsène-Ploetze F (2013) Bacterial metabolism of environmental arsenic—mechanisms and biotechnological applications. Appl Microbiol Biotechnol 97:3827–3841
Lee J-U, Kim S-M, Kim K-W, Kim IS (2005) Microbial removal of uranium in uranium-bearing black shale. Chemosphere 59:147–154. doi:10.1016/j.chemosphere.2004.10.006
Li Q, Ding D, Sun J, Wang Q, Hu E, Shi W, Ma L, Guo X, Liu X (2014) Community dynamics and function variation of a defined mixed bioleaching acidophilic bacterial consortium in the presence of fluoride. Ann Microbiol 65:121–128. doi:10.1007/s13213-014-0843-x
Li Q, Ren Y, Qiu G, Li N, Liu H, Dai Z, Fu X, Shen L, Liang Y, Yin H (2011) Insights into the pH up-shift responsive mechanism of Acidithiobacillus ferrooxidans by microarray transcriptome profiling. Folia Microbiol 56:439–451
Liang Y, Van Nostrand JD, Wang J, Zhang X, Zhou J, Li G (2009) Microarray-based functional gene analysis of soil microbial communities during ozonation and biodegradation of crude oil. Chemosphere 75:193–199
Luo H, Shen L, Yin H, Li Q, Chen Q, Luo Y, Liao L, Qiu G, Liu X (2009) Comparative genomic analysis of Acidithiobacillus ferrooxidans strains using the A. ferrooxidans ATCC 23270 whole-genome oligonucleotide microarray. Can J Microbiol 55:587–598. doi:10.1139/W08-158
Ma LY, Li Q, Xiao YH, Wang QL, Yin HQ, Liang YL, Qiu GZ, Liu XD (2013) Comparative study of fluoride-tolerance of five typical bioleaching microorganisms. Adv Mat Res 825:214–218
Marquis RE, Clock SA, Mota-Meira M (2003) Fluoride and organic weak acids as modulators of microbial physiology. FEMS Microbiol Rev 26:493–510
Matin A, Rittenberg SC (1971) Enzymes of carbohydrate metabolism in Thiobacillus species. J Bacteriol 107:179–186
Pandey B (2013) Microbial processing of apatite rich low grade Indian uranium ore in bioreactor. Bioresour Technol 128:619–623
Parsonage D, Singh P, Nikoloski AN (2014) Adverse effects of fuoride on hydrometallurgical operations. Miner Process Extr Metall Rev 35:44–65
Peng ZJ, Yu RL, Qiu GZ, Qin WQ, Gu GH, Wang QL, Li Q, Liu XD (2013) Really active form of fluorine toxicity affecting Acidithiobacillus ferrooxidans activity in bioleaching uranium. T Nonferr Metal Soc 23:812–817. doi:10.1016/s1003-6326(13)62533-9
Pham ALT, Lee C, Doyle FM, Sedlak DL (2009) A silica-supported iron oxide catalyst capable of activating hydrogen peroxide at neutral pH values. Environ Sci Technol 43:8930–8935
Qihou L, Nuo L, Xueduan L, Zhijun Z, Qian L, Yun F, Xiangru F, Xian F, Yi L, Huaqun Y (2012) Characterization of the acid stress response of Acidithiobacillus ferrooxidans ATCC 23270 based on the method of microarray. J Biol Res 17:3–15
Qiu G, Li Q, Yu R, Sun Z, Liu Y, Chen M, Yin H, Zhang Y, Liang Y, Xu L (2011) Column bioleaching of uranium embedded in granite porphyry by a mesophilic acidophilic consortium. Bioresour Technol 102:4697–4702
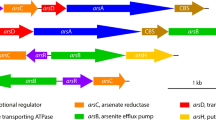
Quatrini R, Jedlicki E, Holmes DS (2005) Genomic insights into the iron uptake mechanisms of the biomining microorganism Acidithiobacillus ferrooxidans. J Ind Microbiol Biotechnol 32:606–614
Raddadi N, Cherif A, Daffonchio D, Neifar M, Fava F (2015) Biotechnological applications of extremophiles, extremozymes and extremolytes. Appl Microbiol Biotechnol 99:7907–7913
Rashidi A, Roosta-Azad R, Safdari S (2014) Optimization of operating parameters and rate of uranium bioleaching from a low-grade ore. J Radioanal Nucl Chem 301:341–350
Rawlings DE, Johnson DB (2007) The microbiology of biomining: development and optimization of mineral-oxidizing microbial consortia. Microbiology 153:315–324
Razzell W, Trussell P (1963) Isolation and properties of an iron-oxidizing Thiobacillus. J Bacteriol 85:595–603
Rodrigues MLM (2015) Biolixiviação de cobre com micro-organismos mesófilos e termófilos moderados: sulfetos secundários contendo flúor e placas de circuito impresso. Universidade Federal de Ouro Preto, Ouro Preto
Silverman MP, Lundgren DG (1959) Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans: I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol 77:642
Smoot LM, Smoot JC, Graham MR, Somerville GA, Sturdevant DE, Migliaccio CAL, Sylva GL, Musser JM (2001) Global differential gene expression in response to growth temperature alteration in group A Streptococcus. Proc Natl Acad Sci USA 98:10416–10421
Stepanauskas R, Glenn TC, Jagoe CH, Tuckfield RC, Lindell AH, McArthur J (2005) Elevated microbial tolerance to metals and antibiotics in metal-contaminated industrial environments. Environ Sci Technol 39:3671–3678
Stintzi A (2003) Gene expression profile of Campylobacter jejuni in response to growth temperature variation. J Bacteriol 185:2009–2016
Sun J, Feng X, Liang D, Duan Y, Lei H (2011) Down-regulation of energy metabolism in Alzheimer’s disease is a protective response of neurons to the microenvironment. J Alzheimer’s Dis 28:389–402
Suzuki I, Lee D, Mackay B, Harahuc L, Oh JK (1999) Effect of various ions, pH, and osmotic pressure on oxidation of elemental sulfur by Thiobacillus thiooxidans. Appl Environ Microbiol 65:5163–5168
Valdes J, Pedroso I, Quatrini R, Dodson RJ, Tettelin H, Blake RC, Eisen JA, Holmes DS (2008) Acidithiobacillus ferrooxidans metabolism: from genome sequence to industrial applications. BMC Genom 9:597
Veloso TC, Sicupira LC, Rodrigues IC, Silva LA, Leão VA (2012) The effects of fluoride and aluminum ions on ferrous-iron oxidation and copper sulfide bioleaching with Sulfobacillus thermosulfidooxidans. Biochem Eng J 62:48–55
Vera M, Schippers A, Sand W (2013) Progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation—part A. Appl Microbiol Biotechnol 97:7529–7541
Wang Q, Qiu G (2011) Study on bacteria domestication and application of heap leaching in uranium mine. In: Remote sensing, environment and transportation engineering (RSETE), International Conference on, 2011 IEEE, pp 8522–8525
Watkin E, Zammit C (2016) Adaption to extreme acidity and osmotic stress. In: Quatrini R, Johnson D (eds) Acidophiles: life in extremely acidic environments. Caister Academic Press, Norfolk
Wen JK, Chen BW, Shang H, Zhang GC (2016) Research progress in biohydrometallurgy of rare metals and heavy nonferrous metals with an emphasis on China. Rare Met 35:433–442. doi:10.1007/s12598-016-0739-y
Xiong J, He Z, Van Nostrand JD, Luo G, Tu S, Zhou J, Wang G (2012) Assessing the microbial community and functional genes in a vertical soil profile with long-term arsenic contamination. PLoS One 7:e50507
Xu M, Zhang Q, Xia C, Zhong Y, Sun G, Guo J, Yuan T, Zhou J, He Z (2014) Elevated nitrate enriches microbial functional genes for potential bioremediation of complexly contaminated sediments. ISME J 8:1932–1944
Xu Y, Yin H, Jiang H, Liang Y, Guo X, Ma L, Xiao Y, Liu X (2013) Comparative study of nickel resistance of pure culture and co-culture of Acidithiobacillus thiooxidans and Leptospirillum ferriphilum. Arch Microbiol 195:637–646
Acknowledgment
This work was supported by the National Natural Science Foundation of China (NSFC 31570113) and Fundamental Research Funds for the Central Universities of Central South University (2016zzts110).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10295_2016_1827_MOESM1_ESM.tif
Fig. S1 Differentially expressed genes grouped by functional classification according to the TIGR A. ferrooxidans ATCC 23270 genome database. Columns: a biosynthesis of cofactors, prosthetic groups, and carriers; b central intermediary metabolism; c mobile and extrachromosomal element functions; d amino acid biosynthesis; e cell envelope; f cellular processes; g DNA metabolism; h energy metabolism; i fatty acid and phospholipid metabolism; j protein fate; k protein synthesis; l purines, pyrimidines, nucleosides, and nucleotides; m regulatory functions; n signal transduction; o transcription; p transport and binding proteins; q hypothetical Proteins-Conserved; r hypothetical Proteins; s unknown functions (TIFF 335 kb)
10295_2016_1827_MOESM2_ESM.tif
Fig. S2 The heatmap exhibits the four co-expression clusters of 22 genes. Red and blue represent co-expression values as 1 and −1, respectively. Genes: 1 transglutaminase-like domain protein (AFE0202); 2 conserved hypothetical protein (AFE0381); 3 ATP-dependent DNA helicase RecQ (AFE0903); 4 conserved hypothetical protein (AFE1091); 5 PIN domain protein (AFE0126); 6 hypothetical protein (AFE1867); 7 transcriptional regulator, GntR family (AFE0811); 8 hypothetical protein (AFE1300); 9 drug resistance transporter, EmrB/QacA family (AFE0913); 10 hypothetical protein (AFE0677); 11 glycogen synthase (AFE0427); 12 transposon, transposition protein B, putative (AFE0597); 13 amino acid permease family protein (AFE0645); 14 hypothetical protein (AFE0868); 15 drug resistance transporter, EmrB/QacA family (AFE2124); 16 PQQ enzyme repeat domain protein (AFE0847); 17 GTP-binding protein (AFE0926); 18 xylulose-5-phosphate/fructose-6-phosphate phosphoketolase (AFE1041); 19 outer membrane toxin secretion efflux protein, putative (AFE0929); 20 major facilitator family transporter (AFE0821); 21 hypothetical protein (AFE2209); 22 ubiquinol-cytochrome c reductase, cytochrome b subunit (AFE0375) (TIFF 1147 kb)
Rights and permissions
About this article
Cite this article
Ma, L., Li, Q., Shen, L. et al. Insights into the fluoride-resistant regulation mechanism of Acidithiobacillus ferrooxidans ATCC 23270 based on whole genome microarrays. J Ind Microbiol Biotechnol 43, 1441–1453 (2016). https://doi.org/10.1007/s10295-016-1827-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-016-1827-6