Abstract
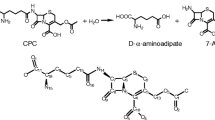
Penicillin acylases are enzymes employed by the pharmaceutical industry for the manufacture of semi-synthetic penicillins. There is a continuous demand for thermostable and alkalophilic enzymes in such applications. We have carried out a computational analysis of known penicillin G acylases (PGAs) in terms of their thermostable nature using various protein-stabilizing factors. While the presence of disulfide bridges was considered initially to screen putative thermostable PGAs from the database, various other factors such as high arginine to lysine ratio, less content of thermolabile amino acids, presence of proline in β-turns, more number of ion-pair and other non-bonded interactions were also considered for comparison. A modified consensus approach designed could further identify stabilizing residue positions by site-specific comparison between mesostable and thermostable PGAs. A most likely thermostable enzyme identified from the analysis was PGA from Paracoccus denitrificans (PdPGA). This was cloned, expressed and tested for its thermostable nature using biochemical and biophysical experiments. The consensus site-specific sequence-based approach predicted PdPGA to be more thermostable than Escherichia coli PGA, but not as thermostable as the PGA from Achromobacter xylosoxidans. Experimental data showed that PdPGA was comparatively less thermostable than Achromobacter xylosoxidans PGA, although thermostability factors favored a much higher stability. Despite being mesostable, PdPGA being active and stable at alkaline pH is an advantage. Finally, several residue positions could be identified in PdPGA, which upon mutation selectively could improve the thermostability of the enzyme.



Similar content being viewed by others
References
Arroyo M, de la Mata I, Acebal C, Castillon MP (2003) Biotechnological applications of penicillin acylases: state-of-the-art. Appl Microbiol Biotechnol 60:507–514. doi:10.1007/s00253-002-1113-6
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The Protein Data Bank. Nucleic Acids Res 28:235–242
Blundell TL, Elliott G, Gardner SP, Hubbard T, Islam S, Johnson M, Mantafounis D, Murray-Rust P, Overington J, Pitts JE, Sali A, Sibanda BL, Singh J, Sternberg MJE, Sutcliffe MJ, Thornton JM, Travers P (1989) Protein Engineering and Design. Philosophical Trans Roy Soc Lond B Biol Sci 324:447–460
Cai G, Zhu S, Yang S, Zhao G, Jiang W (2004) Cloning, overexpression, and characterization of a novel thermostable penicillin G acylase from Achromobacter xylosoxidans: probing the molecular basis for its high thermostability. Appl Environ Microbiol 70:2764–2770
Capriotti E, Fariselli P, Casadio R (2005) I-Mutant2. 0: predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Res 33:W306–W310
Chilov GG, Moody HM, Boesten WHJ, Å vedas VK (2003) Resolution of (RS)-phenylglycinonitrile by penicillin acylase-catalyzed acylation in aqueous medium. Tetrahedron: Asymmetry 14:2613-2617. http://dx.doi.org/10.1016/S0957-4166(03)00523-8
Duggleby HJ, Tolley SP, Hill CP, Dodson EJ, Dodson G, Moody PC (1995) Penicillin acylase has a single-amino-acid catalytic centre. Nature 373:264–268. doi:10.1038/373264a0
Erarslan A, Kocer H (1992) Thermal inactivation kinetics of penicillin G acylase obtained from a mutant derivative of Escherichia coli ATCC 11105. J Chem Technol Biotechnol 55:79–84
Fuganti C, Grasselli P, Casati P (1986) Immobilized penicillin acylase: application to the synthesis of the dipeptide aspartame. Tetrahedron Lett 27:3191–3194
Fuganti C, Grasselli P, Seneci PF, Servi S, Casati P (1986) Immobilized benzylpenicillin acylase: application to the synthesis of optically active forms of carnitin and propranalol. Tetrahedron Lett 27:2061–2062
Guerois R, Nielsen JE, Serrano L (2002) Predicting changes in the stability of proteins and protein complexes: a study of more than 1000 mutations. J Mol Biol 320:369–387
Guranda DT, Khimiuk AI, van Langen LM, van Rantwijk F, Sheldon RA, Å vedas VK (2004) An ‘easy-on, easy-off’ protecting group for the enzymatic resolution of (±)-1-phenylethylamine in an aqueous medium. Tetrahedron: Asymmetry 15:2901-2906. http://dx.doi.org/10.1016/j.tetasy.2004.06.051
Ismail H, Lau RM, van Langen LM, van Rantwijk F, Svedas VK, Sheldon RA (2008) A green, fully enzymatic procedure for amine resolution, using a lipase and a penicillin G acylase. Green Chem 10:415–418. doi:10.1039/b714088f
Kasche V, Lummer K, Nurk A, Piotraschke E, Rieks A, Stoeva S, Voelter W (1999) Intramolecular autoproteolysis initiates the maturation of penicillin amidase from Escherichia coli. Biochim. et Biophys. Acta 1433:76–86
Klock HE, Lesley SA (2009) The Polymerase Incomplete Primer Extension (PIPE) method applied to high-throughput cloning and site-directed mutagenesis. Methods Mol Biol 498:91–103. doi:10.1007/978-1-59745-196-3_6
Lehmann M, Loch C, Middendorf A, Studer D, Lassen SF, Pasamontes L, van Loon AP, Wyss M (2002) The consensus concept for thermostability engineering of proteins: further proof of concept. Protein Eng 15:403–411
Matsumura M, Signor G, Matthews BW (1989) Substantial increase of protein stability by multiple disulphide bonds. Nature 342:291–293. doi:10.1038/342291a0
McVey CE, Walsh MA, Dodson GG, Wilson KS, Brannigan JA (2001) Crystal structures of penicillin acylase enzyme-substrate complexes: structural insights into the catalytic mechanism. J Mol Biol 313:139–150. doi:10.1006/jmbi.2001.5043
Oh B, Kim K, Park J, Yoon J, Han D, Kim Y (2004) Modifying the substrate specificity of penicillin G acylase to cephalosporin acylase by mutating active-site residues. Biochem Biophys Res Commun 319:486–492. doi:10.1016/j.bbrc.2004.05.017
Oinonen C, Rouvinen J (2000) Structural comparison of Ntn-hydrolases. Protein Sci 9:2329–2337. doi:10.1110/ps.9.12.2329
Pace CN, Grimsley GR, Thomson JA, Barnett BJ (1988) Conformational stability and activity of ribonuclease T1 with zero, one, and two intact disulfide bonds. J Biol Chem 263:11820–11825
Polizzi KM, Chaparro-Riggers JF, Vazquez-Figueroa E, Bommarius AS (2006) Structure-guided consensus approach to create a more thermostable penicillin G acylase. Biotechnol J 1:531–536. doi:10.1002/biot.200600029
Pronk S, Pall S, Schulz R, Larsson P, Bjelkmar P, Apostolov R, Shirts MR, Smith JC, Kasson PM, van der Spoel D, Hess B, Lindahl E (2013) GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 29:845–854. doi:10.1093/bioinformatics/btt055
Rawlings ND, Barrett AJ, Bateman A (2012) MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res 40:D343–D350. doi:10.1093/nar/gkr987
Robinson NE (2002) Protein deamidation. Proc Natl Acad Sci USA 99:5283–5288. doi:10.1073/pnas.082102799
Suplatov D, Panin N, Kirilin E, Shcherbakova T, Kudryavtsev P, Svedas V (2014) Computational design of a pH stable enzyme: understanding molecular mechanism of penicillin acylase’s adaptation to alkaline conditions. PLoS One 9:e100643. doi:10.1371/journal.pone.0100643
Suplatov D, Voevodin V, Svedas V (2015) Robust enzyme design: bioinformatic tools for improved protein stability. Biotechnol J 10:344–355. doi:10.1002/biot.201400150
Suzuki Y, Oishi K, Nakano H, Nagayama T (1987) A strong correlation between the increase in number of proline residues and the rise in thermostability of five Bacillus oligo-1,6-glucosidases. Appl Microbiol Biotechnol 26:546–551. doi:10.1007/bf00253030
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Trivedi S, Gehlot HS, Rao SR (2006) Protein thermostability in Archaea and Eubacteria. Genet Mol Res 5:816–827
van Langen LM, van Rantwijk F, Å vedas VK, Sheldon RA (2000) Penicillin acylase-catalyzed peptide synthesis: a chemo-enzymatic route to stereoisomers of 3,6-diphenylpiperazine-2,5-dione. Tetrahedron: Asymmetry 11:1077-1083. http://dx.doi.org/10.1016/S0957-4166(00)00027-6
Varshney NK, Suresh Kumar R, Ignatova Z, Prabhune A, Pundle A, Dodson E, Suresh CG (2012) Crystallization and X-ray structure analysis of a thermostable penicillin G acylase from Alcaligenes faecalis. Acta Crystallographica Section F 68:273–277. doi:10.1107/S1744309111053930
Verhaert RM, Riemens AM, van der Laan JM, van Duin J, Quax WJ (1997) Molecular cloning and analysis of the gene encoding the thermostable penicillin G acylase from Alcaligenes faecalis. Appl Environ Microbiol 63:3412–3418
Vieille C, Zeikus GJ (2001) Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol Mol Biol Rev 65:1–43. doi:10.1128/MMBR.65.1.1-43.2001
Watanabe K, Masuda T, Ohashi H, Mihara H, Suzuki Y (1994) Multiple proline substitutions cumulatively thermostabilize Bacillus cereus ATCC7064 oligo-1,6-glucosidase. Irrefragable proof supporting the proline rule. Eur J Biochem 226:277–283
Acknowledgments
PP, DC and RM thank the Council of Scientific and Industrial Research, New Delhi, for Senior Research Fellowship. SK is DST Ramanujan Fellow. This research was carried out under the CSIR-NCL’s Centre of Excellence in Scientific Computation (CoESC).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Panigrahi, P., Chand, D., Mukherji, R. et al. Sequence and structure-based comparative analysis to assess, identify and improve the thermostability of penicillin G acylases. J Ind Microbiol Biotechnol 42, 1493–1506 (2015). https://doi.org/10.1007/s10295-015-1690-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-015-1690-x