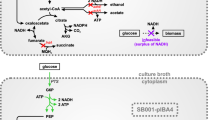
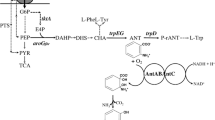
Abstract
Muconic acid (MA) is a promising bulk chemical due to its extensive industrial applications in the production of adipic acid and other valuable, biodegradable intermediates. MA is heretofore mainly produced from petrochemicals by organic reactions which are not environmentally friendly or renewable. Biological production processes provide a promising alternative for MA production. We designed an artificial pathway in Escherichia coli for the biosynthesis of MA using the catechol group of 2,3-dihydroxybenzoate, an intermediate in the enterobactin biosynthesis pathway. This approach consists of two heterologous microbial enzymes, including 2,3-dihydroxybenzoate decarboxylase and catechol 1,2-dioxygenase. The metabolic flow of carbon into the heterologous pathway was optimized by increasing the flux from chorismate through the enterobactin biosynthesis pathway and by regulating the shikimate pathway. Metabolic optimization enabled a concentration of 605.18 mg/L of MA from glucose in a shaking flask culture, a value nearly 484-fold higher than that of the initial recombinant strain. The results indicated that the production of MA from this pathway has the potential for further improvement.








Similar content being viewed by others
References
Alonso-Gutierrez J, Chan R, Batth TS, Adams PD, Keasling JD, Petzold CJ, Lee TS (2013) Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production. Metab Eng 19:33–41
Anderson JJ, Dagley S (1981) Catabolism of tryptophan, anthranilate, and 2, 3-dihydroxybenzoate in Trichosporon cutaneum. J Bacteriol 146:291–297
Bartsch M, Bednarek P, Vivancos PD, Schneider B, von Roepenack-Lahaye E, Foyer CH, Kombrink E, Scheel D, Parker JE (2010) Accumulation of isochorismate-derived 2,3-dihydroxybenzoic 3-O-beta-d-xyloside in arabidopsis resistance to pathogens and ageing of leaves. J Biol Chem 285:25654–25665
Bujnicki RP, Bongaerts JJ, Raeven LJ, Sprenger G, Kuhm A, Takors R Improved biosynthetic production of 2, 3-trans-CHD. EP 1734130 A1
Curran KA, Leavitt JM, Karim AS, Alper HS (2013) Metabolic engineering of muconic acid production in Saccharomyces cerevisiae. Metab Eng 15:55–66
Draths KM, Frost JW (1994) Environmentally compatible synthesis of adipic acid from D-glucose. J Am Chem Soc 116:399–400
Finkelstein J, Antony E, Hingorani MM, O’Donnell M (2003) Overproduction and analysis of eukaryotic multiprotein complexes in Escherichia coli using a dual-vector strategy. Anal Biochem 319:78–87
Franke D, Lorbach V, Esser S, Dose C, Sprenger GA, Halfar M, Thömmes J, Müller R, Takors R, Müller M (2003) (S, S)-2,3-dihydroxy-2,3-dihydrobenzoic acid: microbial access with engineered cells of escherichia coli and application as starting material in natural-product synthesis. Chem Eeu J 9:4188–4196
Gerstle K, Klätschke K, Hahn U, Piganeau N (2012) The small RNA RybA regulates key-genes in the biosynthesis of aromatic amino acids under peroxide stress in E. coli. RNA Biol 9:458–468
Guzik U, Greń I, Hupert-Kocurek K, Wojcieszyńska D (2011) Catechol 1, 2-dioxygenase from rthe new aromatic compounds-degrading Pseudomonas putida strain N6. Int Biodeter Biodegr 65(3):504–512
Jones KL, Kim SW, Keasling JD (2000) Low-copy plasmids can perform as well as or better than high-copy plasmids for metabolic engineering of bacteria. Metab Eng 2(4):328–338
Kikuchi Y, Tsujimoto K, Kurahashi O (1997) Mutational analysis of the feedback sites of phenylalanine-sensitive 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase of Escherichia coli. Appl Environ Microbiol 63:761–762
Lin Y, Sun X, Yuan Q, Yan Y (2014) Extending shikimate pathway for the production of muconic acid and its precursor salicylic acid in Escherichia coli. Metab Eng 23:62–69
Lin Y, Shen X, Yuan Q, Yan Y (2013) Microbial biosynthesis of the anticoagulant precursor 4-hydroxycoumarin. Nat Commun 4:2603. doi:10.1038/ncomms3603
Ma L, Payne SM (2012) AhpC is required for optimal production of enterobactin by Escherichia coli. J Bacteriol 194:6748–6757
Neidhardt FC, Bloch PL, Smith DF (1974) Culture medium for Enterobacteria. J Bacteriol 119:736–747
Neidle EL, Hartnett C, Bonitz S, Ornston LN (1988) DNA sequence of the Acinetobacter calcoaceticus catechol 1, 2-dioxygenase I structural gene catA: evidence for evolutionary divergence of intradiol dioxygenases by acquisition of DNA sequence repetitions. J Bacteriol 170:4874–4880
Niu W, Draths KM, Frost JW (2002) Benzene-free synthesis of adipic acid. Biotechnol Prog 18:201–211
Raymond KN, Dertz EA, Kim SS (2003) Enterobactin: an archetype for microbial iron transport. Proc Natl Acad Sci USA 100:3584–3588
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Santha R, Savithri HS, Rao NA, Vaidyanathan CS (1995) 2,3-Dihydroxybenzoic acid decarboxylase from Aspergillus niger: a novel decarboxylase. Eur J Biochem 230:104–110
Sikora AL, Wilson DJ, Aldrich CC, Blanchard JS (2011) Kinetic and inhibition studies of dihydroxybenzoate-AMP ligase (EntE) from Escherichia coli. Biochemistry 49:3648–3657
Sun X, Lin Y, Huang Q, Yuan Q, Yan Y (2013) A novel muconic acid biosynthesis approach by shunting tryptophan biosynthesis via anthranilate. Appl Environ Microbiol 79:4024–4030
Sun X, Lin Y, Yuan Q, Yan Y (2014) Biological production of muconic acid via a prokaryotic 2,3-dihydroxybenzoic acid decarboxylase. Chem Sus Chem. doi:10.1002/cssc.201402092
van Duuren JB, Wijte D, Leprince A, Karge B, Puchałka J, Wery J, Dos Santos VA, Eggink G, Mars AE (2011) Generation of a catR deficient mutant of P. putida KT2440 that produces cis, cis-muconate from benzoate at high rate and yield. J Biotechnol 156:163–172
van Duuren JB, Wittmann C (2014) Bioprocessing of renewable resources to commodity bioproducts. In: Bisaria VS, Kondo A (eds) First and second generation production of bio-adipic acid, 1st edn. Wiley, New York, pp 519–540
Weber C, Brückner C, Weinreb S, Lehr C, Essl C, Boles E (2012) Biosynthesis of cis, cis-muconic acid and its aromatic precursors, catechol and protocatechuic acid, from renewable feedstocks by Saccharomyces cerevisiae. Appl Environ Microbiol 78:8421–8430
Wu CM, Lee TH, Lee SN, Lee YA, Wu JY (2004) Microbial synthesis of cis, cis-muconic acid by Sphingobacterium sp. GCG generated from effluent of a styrene monomer (SM) production plant. Enzyme Microb Technol 35:598–604
Wu J, Du G, Zhou J, Chen J (2013) Metabolic engineering of Escherichia coli for (2S)-pinocembrin production from glucose by a modular metabolic strategy. Metab Eng 16:48–55
Xie NZ, Liang H, Huang RB, Xu P (2014) Biotechnological production of muconic acid: current status and future prospects. Biotechnol Adv 32:615–622
Acknowledgments
This work was supported by a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the 111 Project (No. 111-2-06), and the Jiangsu province “Collaborative Innovation Center for Advanced Industrial Fermentation” industry development program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Zheng, P. Muconic acid production from glucose using enterobactin precursors in Escherichia coli . J Ind Microbiol Biotechnol 42, 701–709 (2015). https://doi.org/10.1007/s10295-014-1581-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1581-6