Abstract
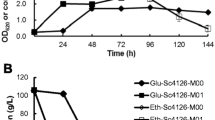
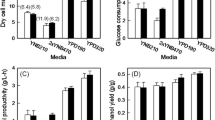
Excessive oxidative stress poses significant damage to yeast cells during fermentation process, and finally affects fermentation efficiency and the quality of products. In this paper, global transcription machinery engineering was employed to elicit Saccharomyces cerevisiae phenotypes of higher tolerance against oxidative stress caused by H2O2. Two strains from two plasmid-based mutagenesis libraries (Spt15 and Taf25), which exhibited significant increases in oxidative stress tolerance, were successfully isolated. At moderate H2O2 shock (≤3.5 mM), a positive correlation was found between the outperformance in cell growth of the oxidation-tolerate strains and H2O2 concentration. Several mutations were observed in the native transcription factors, which resulted in a different transcriptional profile compared with the control. Catalase and superoxide dismutase activities of the two mutants increased under H2O2 stress conditions. Fermentation experiments revealed that the mutant strain taf25-3 has a shorter lag phase compared to the control one, indicating that taf25-3 had improved adaptation ability to H2O2-induced oxidative stress and higher fermentation efficiency. Our study demonstrated that several amino acid substitutions in general transcription factors (Spt15 and Taf25) could modify the cellular oxidation defense systems and improve the anti-oxidation ability of S. cerevisiae. It could make the industrial ethanol fermentation more efficient and cost-effective by using the strain of higher stress tolerance.







Similar content being viewed by others
References
Abdulrehman D, Monteiro PT, Teixeira MC, Mira NP, Lourenco AB, dos Santos SC, Cabrito TR, Francisco AP, Madeira SC, Aires RS, Oliveira AL, Correia IS, Freitas AT (2011) YEASTRACT: providing a programmatic access to curated transcriptional regulatory associations in Saccharomyces cerevisiae through a web services interface. Nucleic Acids Res 39:136–140
Allen S, Clark W, McCaffery JM, Cai Z, Lanctot A, Slininger P, Liu ZL, Gorsich S (2010) Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisiae. Biotechnol Biofuels 3:2
Alper H, Jin YS, Moxley JF, Stephanopoulos G (2005) Identifying gene targets for the metabolic engineering of lycopene biosynthesis in Escherichia coli. Metab Eng 7:155–164
Alper H, Moxley J, Nevoigt E, Fink GR, Stephanopoulos G (2006) Engineering yeast transcription machinery for improved ethanol tolerance and production. Science 314:1565–1568
Alper H, Stephanopoulos G (2007) Global transcription machinery engineering: a new approach for improving cellular phenotype. Metab Eng 9:258–267
Attfield PV (1997) Stress tolerance: the key to effective strains of industrial baker’s yeast. Nat Biotechnol 15:1351–1357
Boundy-Mills K (2012) Yeast culture collections of the world: meeting the needs of industrial researchers. J Ind Microbiol Biotechnol 39:673–680
Casey E, Sedlak M, Ho NWY, Mosier NS (2010) Effect of acetic acid and pH on the cofermentation of glucose and xylose to ethanol by a genetically engineered strain of Saccharomyces cerevisiae. FEMS Yeast Res 10:385–393
Costa V, Quintanilha A, Moradas-Ferreira P (2007) Protein oxidation, repair mechanisms and proteolysis in Saccharomyces cerevisiae. IUBMB Life 59:293–298
Dukan S, Nyström T (1998) Bacterial senescence: stasis results in increased and differential oxidation of cytoplasmic proteins leading to developmental induction of the heat shock regulon. Genes Dev 12:3431–3441
Farr SB, Kogoma T (1991) Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev 55:561–585
Fierro-Risco J, Rincón A, Benítez T, Codón A (2013) Overexpression of stress-related genes enhances cell viability and velum formation in Sherry wine yeasts. Appl Microbiol Biotechnol 97:6867–6881
Kim YC, Geiger JH, Hahn S, Sigler PB (1993) Crystal-structure of a yeast TBP-box complex. Nature 365:512–520
Landolfo S, Politi H, Angelozzi D, Mannazzu I (2008) ROS accumulation and oxidative damage to cell structures in Saccharomyces cerevisiae wine strains during fermentation of high-sugar-containing medium. Biochimica et Biophysica Acta (BBA)-Gen Subj 1780:892–898
Lanza AM, Alper HS (2011) Global strain engineering by mutant transcription factors. Methods Mol Biol 765:253–274
Lelandais G, Tanty V, Geneix C, Etchebest C, Jacq C, Devaux F (2008) Genome adaptation to chemical stress: clues from comparative transcriptomics in Saccharomyces cerevisiae and Candida glabrata. Genome Biol 9:164
Liu H, Liu K, Yan M, Xu L, Ouyang P (2011) gTME for improved adaptation of Saccharomyces cerevisiae to corn cob acid hydrolysate. Appl Biochem Biotechnol 164:1150–1159
Liu ZL (2006) Genomic adaptation of ethanologenic yeast to biomass conversion inhibitors. Appl Microbiol Biotechnol 73:27–36
Liu ZL, Slininger PJ, Dien BS, Berhow MA, Kurzman CP, Gorsich SW (2004) Adaptive response of yeasts to furfural and 5-hydroxymethylfurfural and new chemical evidence for HMF conversion to 2,5-bis-hydroxymethylfuran. J Ind Microbiol Biotechnol 31:345–352
Lushchak VI, Bagnyukova TV, Husak VV, Luzhna LI, Lushchak OV, Storey KB (2005) Hyperoxia results in transient oxidative stress and an adaptive response by antioxidant enzymes in goldfish tissues. Int J Biochem Cell Biol 37:1670–1680
Ma M, Liu ZL (2010) Comparative transcriptome profiling analyses during the lag phase uncover YAP1, PDR1, PDR3, RPN4, and HSF1 as key regulatory genes in genomic adaptation to the lignocellulose derived inhibitor HMF for Saccharomyces cerevisiae. BMC Genom 11:660
Mosier N, Wyman C, Dale B, Elander R, Lee Y-Y, Holtzapple M, Ladisch M (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96:673–686
Olofsson K, Bertilsson M, Liden G (2008) A short review on SSF-an interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol Biofuels 1:7
Patikoglou GA, Kim JL, Sun L, Yang SH, Kodadek T, Burley SK (2005) TATA element recognition by the TATA box-binding protein has been conserved throughout evolution. Genes Dev 13:3217–3230
Rodríguez-Porrata B, Carmona-Gutierrez D, Reisenbichler A, Bauer M, Lopez G, Escoté X, Mas A, Madeo F, Cordero-Otero R (2012) Sip18 hydrophilin prevents yeast cell death during desiccation stress. J Appl Microbiol 112:512–525
Storz G, Christman MF, Sies H, Ames BN (1987) Spontaneous mutagenesis and oxidative damage to DNA in Salmonella typhimurium. Proc Natl Acad Sci USA 84:8917–8921
Thomas MC, Chiang CM (2006) The general transcription machinery and general cofactors. Crit Rev Biochem Mol Biol 41:105–178
Yang J, Bae JY, Lee YM, Kwon H, Moon HY, Kang HA, Yee SB, Kim W, Choi W (2011) Construction of Saccharomyces cerevisiae strains with enhanced ethanol tolerance by mutagenesis of the TATA-binding protein gene and identification of novel genes associated with ethanol tolerance. Biotechnol Bioeng 108:1776–1787
Acknowledgments
The authors thank Dr. Michael R. Ladisch, the Distinguished Professor and Director of the Laboratory of Renewable Resources Engineering (LORRE) at Purdue University for his internal review of this manuscript. The authors also acknowledge support from the National Natural Science Foundation of China (31101237), The Earmarked Fund for Modern Agro-Industry Technology Research System, China (nycytx-30-ch-03), and Innovation Fund for Graduate Student of China Agricultural University (2012YJ079).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, H., Li, J., Han, B. et al. Improvement of oxidative stress tolerance in Saccharomyces cerevisiae through global transcription machinery engineering. J Ind Microbiol Biotechnol 41, 869–878 (2014). https://doi.org/10.1007/s10295-014-1421-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1421-8