Abstract
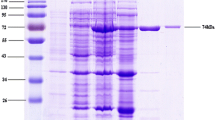
Enhancing the production of α-cyclodextrin glycosyltransferase (α-CGTase) is a key aim in α-CGTase industries. Here, the mature α-cgt gene from Paenibacillus macerans JFB05-01 was redesigned with systematic codon optimization to preferentially match codon frequencies of Escherichia coli without altering the amino acid sequence. Following synthesis, codon-optimized α-cgt (coα-cgt) and wild-type α-cgt (wtα-cgt) genes were cloned into pET-20b(+) and expressed in E. coli BL21(DE3). The total protein yield of the synthetic gene was greater than wtα-cgt expression (1,710 mg L−1) by 2,520 mg L−1, with the extracellular enzyme activity being improved to 55.3 U mL−1 in flask fermentation. ΔG values at -3 to +50 of the pelB site of both genes were −19.10 kcal mol−1. Functionally, coα-CGTase was equally as effective as wtα-CGTase in forming α-cyclodextrin (α-CD). These findings suggest that preferred codon usage is advantageous for translational efficiency to increase protein expression. Finally, batch fermentation was applied, and the extracellular coα-CGTase enzyme activity was 326 % that of wtα-CGTase. The results suggest that codon optimization is a reasonable strategy to improve the yield of α-CGTase for industrial application.




Similar content being viewed by others
References
Angov E (2011) Codon usage: nature’s roadmap to expression and folding of proteins. Biotechnol J 6:650–659
Angov E, Hillier CJ, Kincaid RL, Lyon JA (2008) Heterologous protein expression is enhanced by harmonizing the codon usage frequencies of the target gene with those of the expression host. PLoS ONE 3:e2189
Bar A, Krul CAM, Jonker D, de Vogel N (2004) Safety evaluation of an α-cyclodextrin glycosyltranferase preparation. Regul Toxicol Pharm 39:S47–S56
Burgess-Brown NA, Sharma S, Sobott F, Loenarz C, Oppermann U, Gileadi O (2008) Codon optimization can improve expression of human genes in Escherichia coli: a multi-gene study. Protein Expres Purif 59:94–102
Cheng J, Wu D, Chen S, Chen J, Wu J (2011) High-level extracellular production of α-cyclodextrin glycosyltransferase with recombinant Escherichia coli BL21 (DE3). J Agric Food Chem 59:3797–3802
Chowdhury DR, Angov E, Kariuki T, Kumar N (2009) A potent malaria transmission blocking vaccine based on codon harmonized full length Pfs48/45 expressed in Escherichia coli. PLoS ONE 4:e6352
Ding RR, Li ZF, Chen S, Wu D, Wu J, Chen J (2010) Enhanced secretion of recombinant α-cyclodextrin glucosyltransferase from E. coli by medium additives. Process Biochem 45:880–886
Go YH, Kim TK, Lee KW, Lee YH (2007) Functional characteristics of cyclodextrin glucanotransferase from alkalophilic Bacillus sp BL-31 highly specific for intermolecular transglycosylation of bioflavonoids. J Microbiol Biotechnol 17:1550–1553
Gustafsson C, Govindarajan S, Minshull J (2004) Codon bias and heterologous protein expression. Trends Biotechnol 22:346–353
Gvritishvili AG, Leung KW, Tombran-Tink J (2010) Codon preference optimization increases heterologous PEDF expression. PLoS ONE 5:e15056
Han RZ, Liu L, Li JH, Du GC, Chen J (2012) Functions, applications and production of 2-O-d-glucopyranosyl-l-ascorbic acid. Appl Microbiol Biotechnol 95:313–320
Huang YK, Chen YS, Mo DL, Cong PQ, He ZY (2012) Attenuated secretion of the thermostable xylanase xynB from Pichia pastoris using synthesized sequences optimized from the preferred codon usage in yeast. J Microbiol Biotechnol 22:316–325
Ikemura T (1985) Codon usage and tRNA content in unicellular and multicellular organisms. Mol Biol Evol 2:13–34
Ismail NF, Hamdan S, Mahadi NM, Murad AM, Rabu A, Bakar FD, Klappa P, Illias RM (2011) A mutant L-asparaginase II signal peptide improves the secretion of recombinant cyclodextrin glucanotransferase and the viability of Escherichia coli. Biotechnol Lett 33:999–1005
Jayaraj S, Reid R, Santi DV (2005) GeMS: an advanced software package for designing synthetic genes. Nucleic Acids Res 33:3011–3016
Kodumal SJ, Patel KG, Reid R, Menzella HG, Welch M, Santi DV (2004) Total synthesis of long DNA sequences: synthesis of a contiguous 32-kb polyketide synthase gene cluster. Proc Natl Acad Sci USA 101:15573–15578
Li ZF, Gu ZB, Wang M, Du GC, Wu J, Chen J (2010) Delayed supplementation of glycine enhances extracellular secretion of the recombinant α-cyclodextrin glycosyltransferase in Escherichia coli. Appl Microbiol Biotechnol 85:553–561
Li ZF, Li B, Gu ZB, Du GC, Wu J, Chen J (2010) Extracellular expression and biochemical characterization of α-cyclodextrin glycosyltransferase from Paenibacillus macerans. Carbohydr Res 345:886–892
Li ZF, Li B, Liu ZG, Wang M, Gu ZB, Du GC, Wu J, Chen J (2009) Calcium leads to further increase in glycine-enhanced extracellular secretion of recombinant α-cyclodextrin glycosyltransferase in Escherichia coli. J Agric Food Chem 57:6231–6237
Li ZF, Wang M, Wang F, Gu ZB, Du GC, Wu J, Chen J (2007) γ-Cyclodextrin: a review on enzymatic production and applications. Appl Microbiol Biotechnol 77:245–255
Li ZF, Zhang JY, Sun Q, Wang M, Gu ZB, Du GC, Wu J, Chen J (2009) Mutations of lysine 47 in cyclodextrin glycosyltransferase from Paenibacillus macerans enhance β-cyclodextrin specificity. J Agric Food Chem 57:8386–8391
Li ZF, Zhang JY, Wang M, Gu ZB, Du GC, Li JK, Wu J, Chen J (2009) Mutations at subsite -3 in cyclodextrin glycosyltransferase from Paenibacillus macerans enhancing α-cyclodextrin specificity. Appl Microbiol Biotechnol 83:483–490
Lithwick G, Margalit H (2003) Hierarchy of sequence-dependent features associated with prokaryotic translation. Genome Res 13:2665–2673
Low KO, Muhammad Mahadi N, Abdul Rahim R, Rabu A, Abu Bakar FD, Murad AM, Md Illias R (2011) An effective extracellular protein secretion by an ABC transporter system in Escherichia coli: statistical modeling and optimization of cyclodextrin glucanotransferase secretory production. J Ind Microbiol Biot 38:1587–1597
Ong RM, Goh KM, Mahadi NM, Hassan OH, Rahman RNZRA, Illias RM (2008) Cloning, extracellular expression and characterization of a predominant β-CGTase from Bacillus sp. G1 in E. coli. J Ind Microbiol Biotechnol 35:1705–1714
Plotkin JB, Kudla G (2011) Synonymous but not the same: the causes and consequences of codon bias. Nat Rev Genet 12:32–42
Qi QS, Zimmermann W (2005) Cyclodextrin glucanotransferase: from gene to applications. Appl Microbiol Biotechnol 66:475–485
Kato T, Horikoshi K (1986) Cloning and expression of the Bacillus subtilis NO.313 γ-cyclodextrin forming CGTase gene in Escherichia coli. Agric Biol Chem 50:2161–2162
Tang LX, Jiang RX, Zheng K, Zhu XC (2011) Enhancing the recombinant protein expression of halohydrin dehalogenase HheA in Escherichia coli by applying a codon optimization strategy. Enzyme Microb Technol 49:395–401
Tuller T, Carmi A, Vestsigian K, Navon S, Dorfan Y, Zaborske J, Pan T, Dahan O, Furman I, Pilpel Y (2010) An evolutionarily conserved mechanism for controlling the efficiency of protein translation. Cell 141:344–354
Tuller T, Waldman YY, Kupiec M, Ruppin E (2010) Translation efficiency is determined by both codon bias and folding energy. Proc Natl Acad Sci USA 107:3645–3650
Yang Y, Malten M, Grote A, Jahn D, Deckwer WD (2007) Codon optimized Thermobifida fusca hydrolase secreted by Bacillus megaterium. Biotechnol Bioeng 96:780–794
Zehentgruber D, Lundemo P, Svensson D, Adlercreutz P (2011) Substrate complexation and aggregation influence the cyclodextrin glycosyltransferase (CGTase) catalyzed synthesis of alkyl glycosides. J Biotechnol 155:232–235
Zhang ZC, Li JH, Liu L, Sun J, Hua ZZ, Du GC, Chen J (2011) Enzymatic transformation of 2-O-α-d-glucopyranosyl-l-ascorbic acid (AA-2G) by immobilized α-cyclodextrin glucanotransferase from recombinant Escherichia coli. J Mol Catal B Enzym 68:223–229
Zhang ZC, Li JH, Liu L, Sun J, Hua ZZ, Du GC, Chen J (2011) Enzymatic transformation of 2-O-α-D-glucopyranosyl-L-ascorbic acid by α-cyclodextrin glucanotransferase from recombinant Escherichia coli. Biotechnol Bioproc E 16:107–113
Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31:3406–3415
Acknowledgments
This work was supported by grants from the Major State Basic Research Development Program of China (973 Program, 2012CB720806), the National High Technology Research and Development Program of China (863 Program, SS2012AA023403, 2011AA100905), the Open Project Program of the Key Laboratory of Industrial Biotechnology, Ministry of Education, China (no. KLIB-KF201006), the National Natural Science Foundation of China (31000807), the Natural Science Foundation of Jiangsu Province (BK2011004), the Enterprise-university-research prospective program, Jiangsu Province (BY2009112), the Open Funding Project of the State Key Laboratory of Bioreactor Engineering, the Key Technologies R & D Program of Jiangsu Province, China (BE2011624), and the Fundamental Research Funds for the Central Universities (JUSRP211A29).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
10295_2012_1185_MOESM1_ESM.pdf
Online Resource 1: Comparison of the wild-type (wt, GenBank accession no. AAC04359.1) α-cgt and codon-optimized (co, GenBank accession no. JX412224)α-cgt (* unchanged base pairs). (PDF 87 kb)
Rights and permissions
About this article
Cite this article
Liu, H., Li, J., Du, G. et al. Enhanced production of α-cyclodextrin glycosyltransferase in Escherichia coli by systematic codon usage optimization. J Ind Microbiol Biotechnol 39, 1841–1849 (2012). https://doi.org/10.1007/s10295-012-1185-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-012-1185-y