Abstract
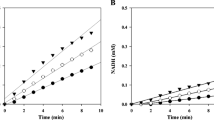
The secretome of Penicillium funiculosum contains two family GH7 enzymes, one of which (designated XynA) has been described as a xylanase. This is unusual because it is the only xylanase in family GH7, which is mainly composed of cellobiohydrolases and endoglucanases, and also because XynA is highly similar to the cellobiohydrolase I from Talaromyces emersonii and Trichoderma reesei (72 and 65 % identity, respectively). To probe this enigma, we investigated the biochemical properties of XynA, notably its activity on xylans and β-d-glucans. A highly pure sample of XynA was obtained and used to perform hydrolysis tests on polysaccharides. These revealed that XynA is 100-fold more active on β-1,4-glucan than on xylan. Likewise, XynA was active on both 4-nitrophenyl-β-d-lactopyranoside (pNP-β-d-Lac) and 4-nitrophenyl-β-d-cellobioside (pNP-cellobiose), which shows that XynA is principally an exo-acting type 1 cellobiohydrolase enzyme that displays 5.2-fold higher performance on pNP-cellobiose than on pNP-β-d-Lac. Finally, analyses performed using cellodextrins as substrate revealed that XynA mainly produced cellobiose (C2) from substrates containing three or more glucosyl subunits, and that C2 inhibits XynA at high concentrations (IC50 C2 = 17.7 μM). Overall, this study revealed that XynA displays typical cellobiohydrolase 1 activity and confirms that the description of this enzyme in public databases should be definitively amended. Moreover, the data provided here complete the information provided by a previous proteomics investigation and reveal that P. funiculosum secretes a complete set of cellulose-degrading enzymes.



Similar content being viewed by others
References
Ahuja SK, Ferreira G, Moreira A (2004) Utilization of enzymes for environmental applications. Crit Rev Biotechnol 24:125–154. doi:10.1080/07388550490493726
Beckham GT, Bomble YJ, Bayer E, Himmel ME, Crowley MF (2011) Applications of computational science for understanding enzymatic deconstruction of cellulose. Curr Opin Biotechnol 22:231–238. doi:10.1016/j.copbio.2010.11.005
Bhat MK (2000) Cellulases and related enzymes in biotechnology. Biotechnol Adv 18:355–383. doi:10.1016/S0734-9750(00)00041-0
Bhat S (2001) Isolation and characterisation of a major cellobiohydrolase (S8) and a major endoglucanase (S11) subunit from the cellulosome of Clostridium thermocellum. Anaerobe 7:171–179. doi:10.1006/anae.2001.0374
Boisset C, Fraschini C, Schülein M, Chanzy H, Henrissat B, Schu M (2000) Imaging the enzymatic digestion of bacterial cellulose ribbons reveals the endo character of the cellobiohydrolase Cel6A from Humicola insolens and its mode of synergy with cellobiohydrolase Cel7A. Appl Environ Microbiol 66:1444–1452. doi:10.1128/AEM.66.4.1444-1452.2000
Boopathy R (1994) Enzyme technology in food and health industries. Indian Food Ind 13:22–31
Cheng Y, Prusoff W (1973) Relationship between the inhibition constant (K i ) and the concentration of inhibitor which causes 50 per cent inhibition (I 50 ) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108. doi:10.1016/0006-2952(73)90196-2
Couturier M, Haon M, Coutinho PM, Henrissat B, Lesage-Meessen L, Berrin JG (2011) Podospora anserina hemicellulases potentiate the Trichoderma reesei secretome for saccharification of lignocellulosic biomass. Appl Environ Microbiol 77:237–246. doi:10.1128/AEM.01761-10
Davies G, Henrissat B (1995) Structures and mechanisms of glycosyl hydrolases. Structure 3:853–859. doi:10.1016/S0969-2126(01)00220-9
Divne C, Ståhlberg J, Teeri T, Jones T (1998) High-resolution crystal structures reveal how a cellulose chain is bound in the 50 Å long tunnel of cellobiohydrolase I from Trichoderma reesei. J Mol Biol 275:309–325. doi:10.1006/jmbi.1997.1437
Eriksson KEL, Blanchette RA, Ander P (1990) Microbial and enzymatic degradation of wood and wood components. In: Microbial and enzymatic degradation of wood and wood components. Springer, New York
Furniss CS, Williamson G, Kroon PA (2005) The substrate specificity and susceptibility to wheat inhibitor proteins of Penicillium funiculosum xylanases from a commercial enzyme preparation. J Sci Food Agric 85:574–582. doi:10.1002/jsfa.1984
Guais O, Borderies G, Pichereaux C, Maestracci M, Neugnot V, Rossignol M, François JM (2008) Proteomics analysis of “Rovabio™ Excel”, a secreted protein cocktail from the filamentous fungus Penicillium funiculosum grown under industrial process fermentation. J Ind Microbiol Biotechnol 35:1659–1668. doi:10.1007/s10295-008-0430-x
Harjunpää V, Teleman A, Koivulaa A, Ruohonen L, Teeri TT, Telemano O, Drakenberg T (1996) Cello-oligosaccharide hydrolysis by cellobiohydrolase II from Trichoderma reesei. Association and rate constants derived from an analysis of progress curves. Eur J Biochem 240:584–591
Henrissat B (1991) A classification of glycosyl hydrolases based on amino-acid sequence similarities. Biochem J 280:309–316
Henrissat B, Davies G (1997) Structural and sequence-based classification of glycoside hydrolases. Curr Opin Struct Biol 7:637–644. doi:10.1016/S0959-440X(97)80072-3
Holtzapple M, Cognata M, Shu Y, Hendrickson C (1990) Inhibition of Trichoderma reesei cellulase by sugars and solvents. Biotechnol Bioeng 36:275–287. doi:10.1002/bit.260360310
Huang L, Forsberg CW (1988) Purification and comparison of the periplasmic and extracellular forms of the cellodextrinase from Bacteroides succinogenes. Appl Environ Microbiol 54:1488–1493
Igarashi K, Samejima M, Eriksson KE (1998) Cellobiose dehydrogenase enhances Phanerochaete chrysosporium cellobiohydrolase I activity by relieving product inhibition. Eur J Biochem 253:101–106
Lee KM (2011) Characterization of cellobiohydrolase from a newly isolated strain of Agaricus arvencis. J Microbiol Biotechnol 21:711–718. doi:10.4014/jmb.1102.02001
Medve J, Lee D, Tjerneld F (1998) Ion-exchange chromatographic purification and quantitative analysis of Trichoderma reesei cellulases cellobiohydrolase I, II and endoglucanase II by fast protein liquid chromatography. Technology 808:153–165. doi:10.1016/S0021-9673(98)00132-0
Merino ST, Cherry J (2007) Progress and challenges in enzyme development for Biomass utilization. Biofuels 108:95–120. doi:10.1007/10_2007_066
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. doi:10.1021/ac60147a030
Muños IG, Ubhayasekera W, Henriksson H, Szabo I, Pettersson G, Johansson G, Mowbray SL, Ståhlberg J (2001) Family 7 cellobiohydrolases from Phanerochaete chrysosporium : crystal structure of the catalytic module of Cel7D (CBH58) at 1.32 Å resolution and homology models of the isozymes. J Molecular Biol 314:1097–1111. doi:10.1006/jmbi.2001.5180
von Ossowski I, Ståhlberg J, Koivula A, Piens K, Becker D, Boer H, Harle R, Harris M, Divne C, Mahdi S, Zhao Y, Driguez H, Claeyssens M, Sinnott ML, Teeri TT (2003) Engineering the exo-loop of Trichoderma reesei cellobiohydrolase, Cel7A. A comparison with Phanerochaete chrysosporium Cel7D. J Mol Biol 333:817–829. doi:10.1016/S0022-2836(03)00881-7
Piens K, Ståhlberg J, Nerinckx W, Teeri TT, Claeyssens M (2004) Structure-reactivity studies of Trichoderma reesei cellobiohydrolase Cel7A. In: Lignocellulose biodegradation, 12: 207–226. Doi:10.1021/bk-2004-0889.ch012
Schmidhalter DR, Canevascini G (1993) Purification and characterisation of 2 exocellobiohydrolases from the brown-rot fungus Coniophora puteana (schum ex-fr) Karst. Arch Biochem Biophys 300:551–558. doi:10.1006/abbi.1993.1076
Schou C, Rasmussen G, Kaltoft M, Henrissat B, Schulein M (1993) Stereochimie, specificity and kinetics of the hydrolysis of reduced cellodextrins by 9 cellulases. Eur J Biochem 217:947–953. doi:10.1111/j.1432-1033.1993.tb18325.x
Schülein M (1997) Enzymatic properties of cellulases from Humicola insolens. J Biotechnol 57:71–81. doi:10.1016/S0168-1656(97)00090-4
Schülein M (2000) Protein engineering of cellulases. Biochim Biophys Acta 1543:239–252. doi:10.1016/S0167-4838(00)00247-8
Sulzenbacher G, Schülein M, Davies G (1997) Structure of the endoglucanase I from Fusarium oxysporum: native, cellobiose, and 3,4-epoxybutyl beta-d-cellobioside-inhibited forms, at 2.3 A resolution. Biochemistry 36:5902–5911. doi:10.1021/bi962963
van Tilbeurgh H, Bhikhabhai R, Pettersson LG, Claeyssens M (1984) Separation of endo- and exo-type cellulases using a new affinity chromatography method. FEBS Lett 169:215–218. doi:10.1016/0014-5793(84)80321-X
Wilson DB (2009) Cellulases and biofuels. Curr Opin Biotechnol 20:295–299. doi:10.1016/j.copbio.2009.05.007
Acknowledgments
The authors would like to thank the Agence National de la Recherche et de la Technologie for financial support accorded to Hélène Texier. Dr. Monsarrat and his team at the IPBS (Institut de Pharmacologie et de Biologie Struturale, Toulouse, France) are thanked for the protein sequence and glycosylation analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Texier, H., Dumon, C., Neugnot-Roux, V. et al. Redefining XynA from Penicillium funiculosum IMI 378536 as a GH7 cellobiohydrolase. J Ind Microbiol Biotechnol 39, 1569–1576 (2012). https://doi.org/10.1007/s10295-012-1166-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-012-1166-1