Abstract
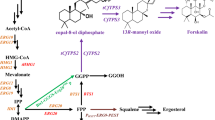
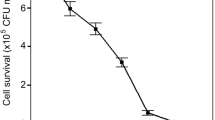
A gene encoding phytoene desaturase (crtI) in the carotenoid biosynthetic pathway of Sphingomonas elodea ATCC 31461, an industrial gellan gum-producing strain, was cloned and identified. This gene is predicted to encode a 492-amino acid protein with significant homology to the phytoene desaturase of other carotenogenic organisms. Knockout of crtI gene blocked yellow carotenoid pigment synthesis and resulted in the accumulation of colorless phytoene, confirming that it encodes phytoene desaturase. Further research indicates that the yield of gellan gum production by crtI gene knockout mutants is almost the same as that by the wild-type strain. In addition, a recovery method based on the colorless fermentation broth of the crtI gene knockout mutant was investigated. Compared to the volume of alcohol for the parent strain, much less alcohol (30%) is required in this recovery process; thus, the costs of downstream purification of gellan gum can be substantially reduced.





Similar content being viewed by others
References
Armstrong G, Alberti M, Leach F, Hearst J (1989) Nucleotide sequence, organization, and nature of the protein products of the carotenoid biosynthesis gene cluster of Rhodobacter capsulatus. Mol Gen Genet 216:254–268
Armstrong GA, Alberti M, Hearst JE (1990) Conserved enzymes mediate the early reactions of carotenoid biosynthesis in nonphotosynthetic and photosynthetic prokaryotes. Proc Natl Acad Sci USA 87:9975–9979
Bajaj IB, Survase SA, Saudagar PS, Singhal RS (2007) Gellan gum: fermentative production, downstream processing and applications. Food Technol Biotechnol 45:341–354
Banik R, Kanari B, Upadhyay S (2000) Exopolysaccharide of the gellan family: prospects and potential. World J Microbiol Biotechnol 16:407–414
Banik RM, Santhiagu A, Upadhyay SN (2007) Optimization of nutrients for gellan gum production by Sphingomonas paucimobilis ATCC 31461 in molasses-based medium using response surface methodology. Bioresour Technol 98:792–797
Bartley GE, Schmidhauser TJ, Yanofsky C, Scolnik PA (1990) Carotenoid desaturases from Rhodobacter capsulatus and Neurospora crassa are structurally and functionally conserved and contain domains homologous to flavoprotein disulfide oxidoreductases. J Biol Chem 265:16020–16024
Ehrenshaft M, Daub M (1994) Isolation, sequence, and characterization of the Cercospora nicotianae phytoene dehydrogenase gene. Appl Environ Microbiol 60:2766–2771
Fialho AM, Monteiro GA, Sa-Correia I (1991) Conjugal transfer of recombinant plasmids into gellan gum-producing and non-producing variants of Pseudomonas elodea ATCC 31461. Lett Appl Microbiol 12:85–87
Fialho AM, Moreira LM, Granja AT, Popescu AO, Hoffmann K, Sa-Correia I (2008) Occurrence, production, and applications of gellan: current state and perspectives. Appl Microbiol Biotechnol 79:889–900
Figurski DH, Helinski DR (1979) Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA 76:1648–1652
Garrido-Fernández J, Maldonado-Barragán A, Caballero-Guerrero B, Hornero-Méndez D, Ruiz-Barba J (2010) Carotenoid production in Lactobacillus plantarum. Int J Food Microbiol 140:34–39
Gay P, Le Coq D, Steinmetz M, Berkelman T, Kado C (1985) Positive selection procedure for entrapment of insertion sequence elements in Gram-negative bacteria. J Bacteriol 164:918–921
Giavasis I, Harvey L, McNeil B (2000) Gellan gum. Crit Rev Biotechnol 20:177–211
Grasdalen H, Smidsrød O (1987) Gelation of gellan gum. Carbohydr Polym 7:371–393
Harding N, Patel Y, Coleman R (2006) Targeted gene deletions for polysaccharide slime formers. US Patent 0199201
Jansson P-E, Lindberg B, Sandford PA (1983) Structural studies of gellan gum, an extracellular polysaccharide elaborated by Pseudomonas elodea. Carbohydr Res 124:135–139
Jenkins C, Andrewes A, McQuade T, Starr M (1979) The pigment of Pseudomonas paucimobilis is a carotenoid (nostoxanthin), rather than a brominated aryl-polyene (xanthomonadin). Curr Microbiol 3:1–4
Kanari B, Banik R, Upadhyay S (2002) Effect of environmental factors and carbohydrate on gellan gum production. Appl Biochem Biotechnol 102:129–140
Kang K, Veeder G (1982) Polysaccharide S-60 and bacterial fermentation process for its preparation. US Patent 4326053
Kang K, Veeder G, Mirrasoul P, Kaneko T, Cottrell I (1982) Agar-like polysaccharide produced by a Pseudomonas species: production and basic properties. Appl Environ Microbiol 43:1086–1091
Kang K, Veeder G, Colegrove G (1983) Deacetylated polysaccharide S-60. US Patent 4385123
Lenz O, Friedrich B (1998) A novel multicomponent regulatory system mediates H2 sensing in Alcaligenes eutrophus. Proc Natl Acad Sci USA 95:12474–12479
Manna B, Gambhir A, Ghosh P (1996) Production and rheological characteristics of the microbial polysaccharide gellan. Lett Appl Microbiol 23:141–145
Miller J (1973) Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, New York
Nampoothiri K, Singhania R, Sabarinath C, Pandey A (2003) Fermentative production of gellan using Sphingomonas paucimobilis. Process Biochem 38:1513–1519
O’ Neill MA, Selvendran RR, Morris VJ (1983) Structure of the acidic extracellular gelling polysaccharide produced by Pseudomonas elodea. Carbohydr Res 124:123–133
Pasamontes L, Hug D, Tessier M, Hohmann HP, Schierle J, van Loon AP (1997) Isolation and characterization of the carotenoid biosynthesis genes of Flavobacterium sp. strain R1534. Gene 185:35–41
Paul F, Morin A, Monsan P (1986) Microbial polysaccharides with actual potential industrial applications. Biotechnol Adv 4:245–259
Sambrook J, Russell D (1986) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York, 999 pp
Sandmann G (1994) Carotenoid biosynthesis in microorganisms and plants. Eur J Biochem 223:7–24
Sutherland I (2001) Microbial polysaccharides from Gram-negative bacteria. Int Dairy J 11:663–674
Takaichi S, Shimada K (1992) Characterization of carotenoids in photosynthetic bacteria. Method Enzymol 213:374–385
Tan G, Gao Y, Shi M, Zhang X, He S, Chen Z et al (2005) SiteFinding-PCR: a simple and efficient PCR method for chromosome walking. Nucleic Acids Res 33:e122
Tonhosolo R, D’Alexandri F, De Rosso V, Gazarini M, Matsumura M, Peres V et al (2009) Carotenoid biosynthesis in intraerythrocytic stages of plasmodium falciparum. J Biol Chem 284:9974–9985
Videira PA, Cortes LL, Fialho AM, Sa-Correia I (2000) Identification of the pgmG gene, encoding a bifunctional protein with phosphoglucomutase and phosphomannomutase activities, in the gellan gum-producing strain Sphingomonas paucimobilis ATCC 31461. Appl Environ Microbiol 66:2252–2258
Wang X, Xu P, Yuan Y, Liu C, Zhang D, Yang Z et al (2006) Modeling for gellan gum production by Sphingomonas paucimobilis ATCC 31461 in a simplified medium. Appl Environ Microbiol 72:3367–3374
West T (2002) Isolation of a mutant strain of Pseudomonas sp. ATCC 31461 exhibiting elevated polysaccharide production. J Ind Microbiol Biotechnol 29:185–188
Yuan C, Morrison N, Clark R (2005): Calcium stable high acyl gellan gum for enhanced colloidal stability in beverages. US Patent 0266138
Acknowledgments
This work was supported by Zhejiang DSM Zhongken Biotechnology Co., Ltd. We thank Dr. Figurski for providing pRK2013, Dr. Lenz for supplying pLO3, and Xinhang Jiang of Zhejiang University for assisting in the HPLC analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Liang Zhu and Xuechang Wu contributed equally to this article.
Rights and permissions
About this article
Cite this article
Zhu, L., Wu, X., Li, O. et al. Cloning and knockout of phytoene desaturase gene in Sphingomonas elodea ATCC 31461 for economic recovery of gellan gum. J Ind Microbiol Biotechnol 38, 1507–1513 (2011). https://doi.org/10.1007/s10295-010-0937-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0937-9