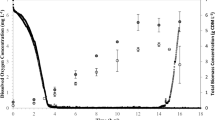
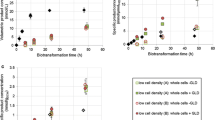
Abstract
Cell physiology is a critical factor determining the efficiency of reactions performed by microbial biocatalysts. In order to develop an efficient biotransformation procedure for the hydroxylation of (S)-limonene to (S)-perillyl alcohol by recombinant Pseudomonas putida cells harboring the cytochrome P450 monooxygenase CYP153A6, physiological parameters were optimized. The previously reported synthesis of (S)-perillyl alcohol by P. putida GPo12 was based on complex and sensitive octane feeding strategies (van Beilen et al. in Appl Environ Microbiol 71:1737–1744, 2005), indicating the pivotal role of cell physiology. In contrast to previous findings, the screening of different carbon sources showed that glycerol and citrate are suitable alternatives to octane allowing high specific limonene hydroxylation activities. The use of P. putida KT2440 as an alternative host strain and citrate as the carbon source improved practical handling and allowed a 7.5-fold increase of the specific activity (to 22.6 U g −1CDW ). In two-liquid-phase biotransformations, 4.3 g of (S)-perillyl alcohol L −1tot were produced in 24 h, representing a sixfold improvement in productivity compared to previously reported results. It is concluded that, for selective cytochrome P450-based hydrocarbon oxyfunctionalizations by means of living microbial cells, the relationship between cell physiology and the target biotransformation is crucial, and that understanding the relationship should guide biocatalyst and bioprocess design.





Similar content being viewed by others
References
Bagdasarian M, Lurz R, Ruckert B, Franklin FC, Bagdasarian MM, Frey J, Timmis KN (1981) Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene 16:237–247
Bar R (1986) Phase toxicity in multiphase biocatalysis. Trends Biotechnol 4:167
Bernhardt R (2006) Cytochromes P450 as versatile biocatalysts. J Biotechnol 124:128–145
Blank LM, Ebert BE, Bühler B, Schmid A (2008) Metabolic capacity estimation of Escherichia coli as a platform for redox biocatalysis: constraint-based modeling and experimental verification. Biotechnol Bioeng 100:1050–1065
Blank LM, Ionidis G, Ebert BE, Bühler B, Schmid A (2008) Metabolic response of Pseudomonas putida during redox biocatalysis in the presence of a second octanol phase. FEBS J 275:5173–5190
Bühler B, Bollhalder I, Hauer B, Witholt B, Schmid A (2003) Chemical biotechnology for the specific oxyfunctionalization of hydrocarbons on a technical scale. Biotechnol Bioeng 82:833–842
Bühler B, Bollhalder I, Hauer B, Witholt B, Schmid A (2003) Use of the two-liquid phase concept to exploit kinetically controlled multistep biocatalysis. Biotechnol Bioeng 81:683–694
Bühler B, Schmid A (2004) Process implementation aspects for biocatalytic hydrocarbon oxyfunctionalization. J Biotechnol 113:183–210
Bühler B, Witholt B, Hauer B, Schmid A (2002) Characterization and application of xylene monooxygenase for multistep biocatalysis. Appl Environ Microbiol 68:560–568
Chastain DE, Sanders Jr. EW, Sanders CC (1992) Using perillyl alcohol to kill bacteria and yeasts. U.S. patent 5,110,832
Cirino PC, Arnold FH (2002) Protein engineering of oxygenases for biocatalysis. Curr Opin Chem Biol 6:130–135
Duetz WA, van Beilen JB, Witholt B (2001) Using proteins in their natural environment: potential and limitations of microbial whole-cell hydroxylations in applied biocatalysis. Curr Opin Biotechnol 12:419–425
Fasan R, Chen MM, Crook NC, Arnold FH (2007) Engineered alkane-hydroxylating cytochrome P450(BM3) exhibiting nativelike catalytic properties. Angew Chem Int Ed Engl 46:8414–8418
Flitsch S, Grogan G, Ashcroft D (2002) Oxidation Reactions. In: Drauz K, Waldmann H (eds) Enzyme catalysis in organic chemistry, 2nd edn. Wiley-VCH Verlag GmbH, Weinheim, Germany, pp 1065–1280
Funhoff EG, Bauer U, Garcia-Rubio I, Witholt B, van Beilen JB (2006) CYP153A6, a soluble P450 oxygenase catalyzing terminal-alkane hydroxylation. J Bacteriol 188:5220–5227
Funhoff EG, Salzmann J, Bauer U, Witholt B, van Beilen JB (2007) Hydroxylation and epoxidation reactions catalyzed by CYP153 enzymes. Enzyme Microb Technol 40:806–812
Gaal T, Bartlett MS, Ross W, Turnbough CL Jr, Gourse RL (1997) Transcription regulation by initiating NTP concentration: rRNA synthesis in bacteria. Science 278:2092–2097
Gillam EM (2007) Extending the capabilities of nature’s most versatile catalysts: directed evolution of mammalian xenobiotic-metabolizing P450s. Arch Biochem Biophys 464:176–186
Gupta A, Myrdal PB (2004) Development of a perillyl alcohol topical cream formulation. Int J Pharm 269:373–383
Hartmans S, van der Werf MJ, de Bont JA (1990) Bacterial degradation of styrene involving a novel flavin adenine dinucleotide-dependent styrene monooxygenase. Appl Environ Microbiol 56:1347–1351
Jensen HP, Sharpless KB (1975) Selenium dioxide oxidation of d-limonene. Reinvestigation. J Org Chem 40:264–265
Julsing MK, Cornelissen S, Bühler B, Schmid A (2008) Heme-iron oxygenases: powerful industrial biocatalysts? Curr Opin Chem Biol 12:177–186
Konopka A (2000) Microbial physiological state at low growth rate in natural and engineered ecosystems. Curr Opin Microbiol 3:244–247
Lageveen RG, Huisman GW, Preusting H, Ketelaar P, Eggink G, Witholt B (1988) Formation of polyesters by Pseudomonas oleovorans—effect of substrates on formation and composition of poly-(R)-3-hydroxyalkanoates and poly-(R)-3-hydroxyalkenoates. Appl Environ Microbiol 54:2924–2932
Leak DJ, Sheldon RA, Woodley JM, Adlercreutz P (2009) Biocatalysts for selective introduction of oxygen. Biocatal Biotransformation 27:1–26
Lebedeva IV, Su ZZ, Vozhilla N, Chatman L, Sarkar D, Dent P, Athar M, Fisher PB (2008) Chemoprevention by perillyl alcohol coupled with viral gene therapy reduces pancreatic cancer pathogenesis. Mol Cancer Ther 7:2042–2050
Lee YW, Lee CH, Kim JD, Lee YY, Row KH (2000) Extraction of perillyl alcohol in Korean orange peel by supercritical CO2. Sep Sci Technol 35:1069–1076
Marshall JA, Sehon CA (1997) Total synthesis of the enantiomer of the furanocembrane rubifolide. J Org Chem 62:4313–4320
Marshall JA, Van Devender EA (2001) Synthesis of (−)-deoxypukalide, the enantiomer of a degradation product of the furanocembranolide pukalide. J Org Chem 66:8037–8041
Nelson KE, Weinel C, Paulsen IT, Dodson RJ, Hilbert H, dos Santos V, Fouts DE, Gill SR, Pop M, Holmes M, Brinkac L, Beanan M, DeBoy RT, Daugherty S, Kolonay J, Madupu R, Nelson W, White O, Peterson J, Khouri H, Hance I, Lee PC, Holtzapple E, Scanlan D, Tran K, Moazzez A, Utterback T, Rizzo M, Lee K, Kosack D, Moestl D, Wedler H, Lauber J, Stjepandic D, Hoheisel J, Straetz M, Heim S, Kiewitz C, Eisen J, Timmis KN, Dusterhoft A, Tummler B, Fraser CM (2002) Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ Microbiol 4:799–808
Omura T, Sato R (1964) The carbon monoxide-binding pigment of liver microsomes. II. Solubilization, purification, and properties. J Biol Chem 239:2379–2385
Opdyke DL (1981) Monographs on fragrance raw materials. Food Cosmet Toxicol 19:237–254
Panke S, Witholt B, Schmid A, Wubbolts MG (1998) Towards a biocatalyst for (S)-styrene oxide production: characterization of the styrene degradation pathway of Pseudomonas sp. strain VLB120. Appl Environ Microbiol 64:2032–2043
Park JB, Bühler B, Panke S, Witholt B, Schmid A (2007) Carbon metabolism and product inhibition determine the epoxidation efficiency of solvent-tolerant Pseudomonas sp. strain VLB120ΔC. Biotechnol Bioeng 98:1219–1229
Peterson JA, Coon MJ (1968) Enzymatic ω-oxidation. III. Purification and properties of rubredoxin, a component of the ω-hydroxylation system of Pseudomonas oleovorans. J Biol Chem 243:329–334
Preusting H, van Houten R, Hoefs A, van Langenberghe EK, Favre-Bulle O, Witholt B (1993) High cell density cultivation of Pseudomonas oleovorans: growth and production of poly(3-hydroxyalkanoates) in two-liquid phase batch and fed-batch systems. Biotechnol Bioeng 41:550–556
Puchalka J, Oberhardt MA, Godinho M, Bielecka A, Regenhardt D, Timmis KN, Papin JA, Martins dos Santos VA (2008) Genome-scale reconstruction and analysis of the Pseudomonas putida KT2440 metabolic network facilitates applications in biotechnology. PLoS Comput Biol 4:e1000210
Ramos JL, Duque E, Huertas MJ, Haidour A (1995) Isolation and expansion of the catabolic potential of a Pseudomonas putida strain able to grow in the presence of high concentrations of aromatic hydrocarbons. J Bacteriol 177:3911–3916
Ruijssenaars HJ, Sperling EM, Wiegerinck PH, Brands FT, Wery J, de Bont JA (2007) Testosterone 15β-hydroxylation by solvent tolerant Pseudomonas putida S12. J Biotechnol 131:205–208
Sakuda Y (1969) The oxidation of limonene with selenium dioxide. Bull Chem Soc Jpn 42:3348–3349
Sambrook J, Russell DW (2001) Molecular cloning. A laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Schneider S, Wubbolts MG, Sanglard D, Witholt B (1998) Biocatalyst engineering by assembly of fatty acid transport and oxidation activities for in vivo application of cytochrome P-450(BM-3) monooxygenase. Appl Environ Microbiol 64:3784–3790
Shimizu N, Mizogughi A, Murakami K, Noge K, Mori N, Nishida R, Kuwahara Y (2006) Synthesis of (+)-(S)-isorobinal together with its antipod, a cyclic monoterpene functioning as the sex pheromone of Rhizoglyphus setosus and its distribution among Astigmata. J Pestic Sci 31:311–315
Sikkema J, de Bont JA, Poolman B (1994) Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem 269:8022–8028
Smits TH, Balada SB, Witholt B, van Beilen JB (2002) Functional analysis of alkane hydroxylases from Gram-negative and Gram-positive bacteria. J Bacteriol 184:1733–1742
Staijen IE, Marcionelli R, Witholt B (1999) The P-alkBFGHJKL promoter is under carbon catabolite repression control in Pseudomonas oleovorans but not in Escherichia coli alk(+) recombinants. J Bacteriol 181:1610–1616
Straathof AJJ, Panke S, Schmid A (2002) The production of fine chemicals by biotransformations. Curr Opin Biotechnol 13:548–556
Sun ZY, Ramsay JA, Guay M, Ramsay BA (2006) Automated feeding strategies for high-cell-density fed-batch cultivation of Pseudomonas putida KT2440. Appl Environ Microbiol 71:423–431
Ueda T, Coon MJ (1972) Enzymatic ω-oxidation. VII. Reduced diphosphopyridine nucleotide-rubredoxin reductase: properties and function as an electron carrier in ω-hydroxylation. J Biol Chem 247:5010–5016
van Beilen JB, Duetz WA, Schmid A, Witholt B (2003) Practical issues in the application of oxygenases. Trends Biotechnol 21:170–177
van Beilen JB, Funhoff EG (2005) Expanding the alkane oxygenase toolbox: new enzymes and applications. Curr Opin Biotechnol 16:308–314
van Beilen JB, Funhoff EG, van Loon A, Just A, Kaysser L, Bouza M, Holtackers R, Rothlisberger M, Li Z, Witholt B (2006) Cytochrome P450 alkane hydroxylases of the CYP153 family are common in alkane-degrading eubacteria lacking integral membrane alkane hydroxylases. Appl Environ Microbiol 72:59–65
van Beilen JB, Holtackers R, Luscher D, Bauer U, Witholt B, Duetz WA (2005) Biocatalytic production of perillyl alcohol from limonene by using a novel Mycobacterium sp. cytochrome P450 alkane hydroxylase expressed in Pseudomonas putida. Appl Environ Microbiol 71:1737–1744
van Beilen JB, Panke S, Lucchini S, Franchini AG, Rothlisberger M, Witholt B (2001) Analysis of Pseudomonas putida alkane-degradation gene clusters and flanking insertion sequences: evolution and regulation of the alk genes. Microbiology (Reading, Engl) 147:1621–1630
van Beilen JB, Penninga D, Witholt B (1992) Topology of the membrane-bound alkane hydroxylase of Pseudomonas oleovorans. J Biol Chem 267:9194–9201
Walton AZ, Stewart JD (2004) Understanding and improving NADPH-dependent reactions by nongrowing Escherichia coli cells. Biotechnol Prog 20:403–411
Weber FJ, de Bont JA (1996) Adaptation mechanisms of microorganisms to the toxic effects of organic solvents on membranes. Biochim Biophys Acta 1286:225–245
Acknowledgments
We gratefully thank Dr. J. B. van Beilen and Prof. Dr. B. Witholt for providing bacterial strains and plasmids. This project was co-financed by the Deutsche Bundesstiftung Umwelt (AZ 20006/855), the ERA-Net project PSYSMO, and the Ministry of Innovation, Science, Research and Technology of North Rhine-Westphalia, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cornelissen, S., Liu, S., Deshmukh, A.T. et al. Cell physiology rather than enzyme kinetics can determine the efficiency of cytochrome P450-catalyzed C–H-oxyfunctionalization. J Ind Microbiol Biotechnol 38, 1359–1370 (2011). https://doi.org/10.1007/s10295-010-0919-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0919-y