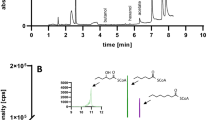
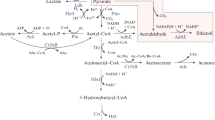
Abstract
Solvent-producing clostridia are well known for their capacity to use a wide variety of renewable biomass and agricultural waste materials for biobutanol production. To investigate the possibility of co-production of a high value chemical during biobutanol production, the Clostridium acetobutylicum riboflavin operon ribGBAH was over-expressed in C. acetobutylicum on Escherichia coli–Clostridium shuttle vector pJIR750. Constructs that either maintained the original C. acetobutylicum translational start codon or modified the start codons of ribG and ribB from TTG to ATG were designed. Riboflavin was successfully produced in both E. coli and C. acetobutylicum using these plasmids, and riboflavin could accumulate up to 27 mg/l in Clostridium culture. Furthermore, the C. acetobutylicum purine pathway was modified by over-expression of the Clostridium purF gene, which encodes the enzyme PRPP amidotransferase. The function of the plasmid pJaF bearing C. acetobutylicum purF was verified by its ability to complement an E. coli purF mutation. However, co-production of riboflavin with biobutanol by use of the purF over-expression plasmid was not improved under the experimental conditions examined. Further rational mutation of the purF gene was conducted by replacement of amino acid codons D302 V and K325Q to make it similar to the feedback-resistant enzymes of other species. However, the co-expression of ribGBAH and purFC in C. acetobutylicum also did not improve riboflavin production. By buffering the culture pH, C. acetobutylicum ATCC 824(pJpGN) could accumulate more than 70 mg/l riboflavin while producing 190 mM butanol in static cultures. Riboflavin production was shown to exert no effect on solvent production at these levels.








Similar content being viewed by others
References
Alvira P, Tomas-Pejo E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861
Atsumi S, Cann AF, Connor MR, Shen CR, Smith KM, Brynildsen MP, Chou KJ, Hanai T, Liao JC (2008) Metabolic engineering of Escherichia coli for 1-butanol production. Metab Eng 10:305–311
Bacher A, Eberhardt S, Eisenreich W, Fischer M, Herz S, Illarionov B, Kis K, Richter G (2001) Biosynthesis of riboflavin. Vitam Horm 61:1–49
Bannam TL, Rood JI (1993) Clostridium perfringens-Escherichia coli shuttle vectors that carry single antibiotic resistance determinants. Plasmid 29:233–235
Beesch SC (1953) Acetone-butanol fermentation of starches. Appl Microbiol 1:85–95
Burgess C, O’Connell-Motherway M, Sybesma W, Hugenholtz J, van Sinderen D (2004) Riboflavin production in Lactococcus lactis: potential for in situ production of vitamin-enriched foods. Appl Environ Microbiol 70:5769–5777
Claassen PA, Budde MA, Lopez-Contreras AM (2000) Acetone, butanol and ethanol production from domestic organic waste by solventogenic clostridia. J Mol Microbiol Biotechnol 2:39–44
Clark SW, Bennett GN, Rudolph FB (1989) Isolation and characterization of mutants of Clostridium acetobutylicum ATCC 824 deficient in acetoacetyl-coenzyme A: acetate/butyrate:coenzyme A-transferase (EC 2.8.3.9) and in other solvent pathway enzymes. Appl Environ Microbiol 55:970–976
Demain AL (1972) Riboflavin oversynthesis. Annu Rev Microbiol 26:369–388
Durre P (2007) Biobutanol: an attractive biofuel. Biotechnol J 2:1525–1534
Durre P (2008) Fermentative butanol production: bulk chemical and biofuel. Ann N Y Acad Sci 1125:353–362
Ezeji TC, Qureshi N, Blaschek HP (2007) Butanol production from agricultural residues: impact of degradation products on Clostridium beijerinckii growth and butanol fermentation. Biotechnol Bioeng 97:1460–1469
Ezeji TC, Qureshi N, Blaschek HP (2004) Butanol fermentation research: upstream and downstream manipulations. Chem Rec 4:305–314
Fassbinder F, Kist M, Bereswill S (2000) Structural and functional analysis of the riboflavin synthesis genes encoding GTP cyclohydrolase II (ribA), DHBP synthase (ribBA), riboflavin synthase (ribC), and riboflavin deaminase/reductase (ribD) from Helicobacter pylori strain P1. FEMS Microbiol Lett 191:191–197
Gamage J, Lam H, Zhang ZS (2010) Bioethanol production from lignocellulosic biomass, a review. J Biobased Mater Bioenergy 4:3–11
Gelfand MS, Mironov AA, Jomantas J, Kozlov YI, Perumov DA (1999) A conserved RNA structure element involved in the regulation of bacterial riboflavin synthesis genes. Trends Genet 15:439–442
Green EM, Boynton ZL, Harris LM, Rudolph FB, Papoutsakis ET, Bennett GN (1996) Genetic manipulation of acid formation pathways by gene inactivation in Clostridium acetobutylicum ATCC 824. Microbiology 142:2079–2086
Hickey JR (1945) Production of riboflavin by butyl alcohol producing bacteria. US patent 2425280
Hümbelin M, Griesser V, Keller T, Schurter W, Haiker M, Hohmann HP, Ritz H, Richter G, Bacher A, van Loon APGM (1999) GTP cyclohydrolase II and 3,4-dihydroxy-2-butanone 4-phosphate synthase are rate-limiting enzymes in riboflavin synthesis of an industrial Bacillus subtilis strain used for riboflavin production. J Ind Microbiol Biotechnol 22:1–7
Yatsishyn VY, Fedorovich DV, Sibirnyi AA (2009) The microbial synthesis of flavin nucleotides: a review. Appl Biochem Biotechnol 45:133–142
Inui M, Suda M, Kimura S, Yasuda K, Suzuki H, Toda H, Yamamoto S, Okino S, Suzuki N, Yukawa H (2008) Expression of Clostridium acetobutylicum butanol synthetic genes in Escherichia coli. Appl Microbiol Biotechnol 77:1305–1316
Jimenez A, Santos MA, Pompejus M, Revuelta JL (2005) Metabolic engineering of the purine pathway for riboflavin production in Ashbya gossypii. Appl Environ Microbiol 71:5743–5751
Johnson JL, Toth J, Santiwatanakul S, Chen JS (1997) Cultures of “Clostridium acetobutylicum” from various collections comprise Clostridium acetobutylicum, Clostridium beijerinckii, and two other distinct types based on DNA–DNA reassociation. Int J Syst Bacteriol 47:420–424
Jones DT, Woods DR (1986) Acetone-butanol fermentation revisited. Microbiol Rev 50:484–524
Knorr B, Schlieker H, Hohmann H-P, Weuster-Botz D (2007) Scale-down and parallel operation of the riboflavin production process with Bacillus subtilis. Biochem Eng J 33:263–274
Lee JY, Jang YS, Lee J, Papoutsakis ET, Lee SY (2009) Metabolic engineering of Clostridium acetobutylicum M5 for highly selective butanol production. Biotechnol J 4:1432–1440
Leviton A (1946) The microbiological synthesis of riboflavin—a theory concerning its inhibition. J Am Chem Soc 68:835–840
Leviton A, Whittier EO (1950) The utilization of whey in the microbiological synthesis of riboflavin. J Dairy Sci 33:402
Martinez I, Zhu J, Lin H, Bennett GN, San KY (2008) Replacing Escherichia coli NAD-dependent glyceraldehyde 3-phosphate dehydrogenase (GAPDH) with a NADP-dependent enzyme from Clostridium acetobutylicum facilitates NADPH dependent pathways. Metab Eng 10:352–359
Marx H, Mattanovich D, Sauer M (2008) Overexpression of the riboflavin biosynthetic pathway in Pichia pastoris. Microb Cell Fact 7:23
Mermelstein LD, Papoutsakis ET (1993) In vivo methylation in Escherichia coli by the Bacillus subtilis phage phi 3T I methyltransferase to protect plasmids from restriction upon transformation of Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 59:1077–1081
Mermelstein LD, Welker NE, Bennett GN, Papoutsakis ET (1992) Expression of cloned homologous fermentative genes in Clostridium acetobutylicum ATCC 824. Biotechnology (NY) 10:190–195
Muchmore CR, Krahn JM, Kim JH, Zalkin H, Smith JL (1998) Crystal structure of glutamine phosphoribosylpyrophosphate amidotransferase from Escherichia coli. Protein Sci 7:39–51
Nair RV, Green EM, Watson DE, Bennett GN, Papoutsakis ET (1999) Regulation of the sol locus genes for butanol and acetone formation in Clostridium acetobutylicum ATCC 824 by a putative transcriptional repressor. J Bacteriol 181:319–330
Pridham TG (1946) Microbial synthesis of riboflavin. Econ Bot 6:185–205
Qureshi N, Blaschek HP (2001) ABE production from corn: a recent economic evaluation. J Ind Microbiol Biotechnol 27:292–297
Sambrook J, Russel, David W (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New york
Sauer U, Hatzimanikatis V, Hohmann HP, Manneberg M, van Loon AP, Bailey JE (1996) Physiology and metabolic fluxes of wild-type and riboflavin-producing Bacillus subtilis. Appl Environ Microbiol 62:3687–3696
Scotcher MC, Bennett GN (2005) SpoIIE regulates sporulation but does not directly affect solventogenesis in Clostridium acetobutylicum ATCC 824. J Bacteriol 187:1930–1936
Scotcher MC, Bennett GN (2008) Activity of abrB310 promoter in wild type and spo0A-deficient strains of Clostridium acetobutylicum. J Ind Microbiol Biotechnol 35:743–750
Shimaoka M, Takenaka Y, Kurahashi O, Kawasaki H, Matsui H (2007) Effect of amplification of desensitized purF and prs on inosine accumulation in Escherichia coli. J Biosci Bioeng 103:255–261
Smith JL, Zaluzec EJ, Wery JP, Niu L, Switzer RL, Zalkin H, Satow Y (1994) Structure of the allosteric regulatory enzyme of purine biosynthesis. Science 264:1427–1433
Stahmann KP, Revuelta JL, Seulberger H (2000) Three biotechnical processes using Ashbya gossypii, Candida famata, or Bacillus subtilis compete with chemical riboflavin production. Appl Microbiol Biotechnol 53:509–516
Steen EJ, Chan R, Prasad N, Myers S, Petzold CJ, Redding A, Ouellet M, Keasling JD (2008) Metabolic engineering of Saccharomyces cerevisiae for the production of n-butanol. Microb Cell Fact 7:36
Switzer RL, Ruppen ME, Bernlohr DA (1982) Inactivation of glutamine: 5-phosphoribosyl 1-pyrophosphate amidotransferase in Bacillus subtilis—oxidation of an essential Fe-S center precedes selective degradation. Biochem Soc Trans 10:322–324
Vitreschak AG, Rodionov DA, Mironov AA, Gelfand MS (2002) Regulation of riboflavin biosynthesis and transport genes in bacteria by transcriptional and translational attenuation. Nucleic Acids Res 30:3141–3151
Zalkin H, Smith JL, Switzer RL (2001) Deregulation of glutamine PRPP amidotransferase activity. US patent 6,204,041
Zhao YS, Hindorff LA, Chuang A, Monroe-Augustus M, Lyristis M, Harrison ML, Rudolph FB, Bennett GN (2003) Expression of a cloned cyclopropane fatty acid synthase gene reduces solvent formation in Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 69:2831–2841
Zhou G, Smith JL, Zalkin H (1994) Binding of purine nucleotides to two regulatory sites results in synergistic feedback inhibition of glutamine 5-phosphoribosylpyrophosphate amidotransferase. J Biol Chem 269:6784–6789
Acknowledgments
This project was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number 2006-35504-17294. Also, we would like to thank Mary Harrison for her assistance for this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, X., Bennett, G.N. Improving the Clostridium acetobutylicum butanol fermentation by engineering the strain for co-production of riboflavin. J Ind Microbiol Biotechnol 38, 1013–1025 (2011). https://doi.org/10.1007/s10295-010-0875-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0875-6