Abstract
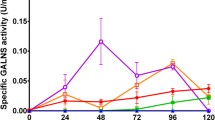
Mucopolysaccharidosis IVA (MPS IVA) is an autosomal recessive disorder caused by N-acetylgalactosamine-6-sulfate sulfatase (GALNS) deficiency. Currently no effective therapies exist for MPS IVA. In this work, production of a recombinant GALNS enzyme (rGALNS) in Escherichia coli BL21 strain was studied. At shake scale, the effect of glucose concentration on microorganism growth, and microorganism culture and induction times on rGALNS production were evaluated. At bench scale, the effect of aeration and agitation on microorganism growth, and culture and induction times were evaluated. The highest enzyme activity levels at shake scale were observed in 12 h culture after 2–4 h induction. At bench scale the highest enzyme activity levels were observed after 2 h induction. rGALNS amounts in inclusion bodies fraction were up to 17-fold higher than those observed in the soluble fraction. However, the highest levels of active enzyme were found in the soluble fraction. Western blot analysis showed the presence of a 50-kDa band, in both soluble and inclusion bodies fractions. These results show for the first time the feasibility and potential of production of active rGALNS in a prokaryotic system for development of enzyme replacement therapy for MPS IVA disease.




Similar content being viewed by others
References
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1999) Short protocols in molecular biology. Wiley, Hoboken
Baker K, Rendall M, Hills A, Hoare M, Freedman R, James D (2001) Metabolic control of recombinant protein N-Glycan processing in NS0 and CHO cells. Biotechnol Bioeng 73:188–202
Baneyx F (1999) Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol 10:411–421
Bernardes GJ, Castagner B, Seeberger PH (2009) Combined approaches to the synthesis and study of glycoproteins. ACS Chem Biol 4:703–713
Bhattacharya SK, Dubey AK (1997) Effects of dissolved oxygen and oxygen mass transfer on overexpression of target gene in recombinant E. coli. Enzyme Microb Technol 20:355–360
Bielicki J, Fuller M, Guo X, Morri C, Hopwood J, Anson D (1995) Expression, purification and characterization of recombinant human N-acetylgalactosamine-6-sulphatase. Biochem J 311:333–339
Braulke T, Bonifacino JS (2009) Sorting of lysosomal proteins. Biochim Biophys Acta 1793:605–614
Çalik P, Yilgör P, Ayhan P, Demir AS (2004) Oxygen transfer effects on recombinant benzaldehyde lyase production. Chem Eng Sci 59:5075–5083
Chu L, Robinson D (2001) Industrial choices for protein production by large-scale cell culture. Curr Opin Biotechnol 12:180–187
Córdoba-Ruiz HA, Poutou-Piñales RA, Echeverri-Peña OY, Algecira-Enciso NA, Landázuri P, Sáenz H, Barrera-Avellaneda LA (2009) Laboratory scale production of the human recombinant iduronate 2-sulfate sulfatase-like from Pichia pastoris. Afr J Biotechnol 8:1786–1792
Cosma M, Pepe P, Annunziata I, Newbold R, Grompe M, Parenti G, Ballabio A (2003) The multiple sulfatase deficiency gene encodes an essential and limiting factor for the activity of sulfatases. Cell 113:445–456
Cromwell ME, Hilario E, Jacobson F (2006) Protein aggregation and bioprocessing. AAPS J 8:E572–E579
Diaz-Ricci J, Hernandez M (2000) Plasmid effects on Escherichia coli metabolism. Crit Rev Biotechnol 20:79–108
Dittmer F, Ulbrich EJ, Hafner A, Schmahl W, Meister T, Pohlmann R, von Figura K (1999) Alternative mechanisms for trafficking of lysosomal enzymes in mannose 6-phosphate receptor-deficient mice are cell type-specific. J Cell Sci 112:1591–1597
Durany O, de Mas C, López-Santín J (2005) Fed-batch production of recombinant fuculose-1-phosphate aldolase in E. coli. Process Biochem 40:707–716
Dvorak-Ewell M, Wendt D, Shroff S, Koppaka V, Christianson T, Vellard M (2009) Functional evaluation of recombinant human N-acetylgalactosamine-6-sulfate sulfatase as enzyme replacement therapy. Mol Genet Metab 96:S22
Graumann K, Premstaller A (2006) Manufacturing of recombinant therapeutic proteins in microbiol systems. Biotechnol J 1:164–186
Greenwald RB, Choe YH, McGuire J, Conover CD (2003) Effective drug delivery by PEGylated drug conjugates. Adv Drug Deliv Rev 55:217–250
Grubb JH, Vogler C, Levy B, Galvin N, Tan Y, Sly WS (2008) Chemically modified b-glucuronidase crosses blood–brain barrier and clears neuronal storage in murine mucopolysaccharidosis VII. Proc Natl Acad Sci U S A 105:2616–2621
Kornfeld S (1987) Trafficking of lysosomal enzymes. FASEB J 1:462–468
Landázuri P, Gunturiz M, Gómez L, Poutou-Piñales RA, Torres A, Echeverri-Peña OY, Sáenz H, Delgado J, Barrera-Avellaneda LA (2003) Expresión transiente de la Iduronato 2 sulfato sulfatasa humana recombinante funcionalmente activa en Escherichia coli. Rev Asoc Col Ciencias Biol 15:33–42
Landázuri P, Poutou-Piñales RA, Acero-Godoy J, Córdoba-Ruiz H, Echeverri-Peña OY, Sáenz H, Delgado J, Barrera-Avellaneda LA (2009) Cloning and shake flask expression of hrIDS-like in Pichia pastoris. Afr J Biotechnol 8:2871–2877
Landgrebe J, Dierks T, Schamidt B, von Figura K (2003) The human SUMF1 gene, required for posttranslational sulfatase modification, defines a new gene family which is conserved from pro- to eukaryotes. Gene 316:47–56
Li R-Y, Cheng C-Y (2009) Investigation of inclusion body formation in recombinant Escherichia coli with a bioimaging system. J Biosci Bioeng 107:512–515
Lin-Chao S, Bremer H (1986) Effect of the bacterial growth rate on replication control of plasmid pBR322 in Escherichia coli. Mol Gen Genet 203:143–149
Luli G, Strohl W (1990) Comparison of growth, acetate production, and acetate inhibition of Escherichia coli strains in batch and fed-batch fermentations. Appl Environ Microbiol 56:1004–1011
Meuwly F, Ruffieux P, Kadouri A, von Stockar U (2007) Packed-bed bioreactors for mammalian cell culture: bioprocess and biomedical applications. Biotechnol Adv 25:45–56
Montaño AM, Sukegawa K, Kato Z, Carrozzo R, Di Natale P, Christensen E, Orii KO, Orii T, Kondo N, Tomatsu S (2007) Effect of ‘attenuated’ mutations in mucopolysaccharidosis IVA on molecular phenotypes of N-acetylgalactosamine-6-sulfate sulfatase. J Inherit Metab Dis 30:758–767
Montaño AM, Tomatsu S, Gottesman G, Smith M, Orii T (2007) International Morquio A registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis 30:165–174
Neufeld E, Muenzer J (2001) The mucopolysaccharidosis. In: Scriver C, Beaudet A, Sly W, Valle D (eds) The metabolic and molecular bases of inherited diseases. McGraw-Hill, New York, pp 3421–3452
Patra AK, Mukhopadhyay R, Mukhija R, Krishnan A, Garg LC, Panda AK (2000) Optimization of inclusion body solubilization and renaturation of recombinant human growth hormone from Escherichia coli. Protein Exp Purif 18:182–192
Phue J-N, Lee SJ, Trinh L, Shiloach J (2008) Modified Escherichia coli B (BL21), a superior producer of plasmid DNA compared with Escherichia coli K (DH5a). Biotechnol Bioeng 101:831–836
Phue J-N, Noronha SB, Hattacharyya R, Wolfe AJ, Shiloach J (2005) Glucose metabolism at high density growth of E. coli B and E. coli K: differencs in metabolic pathways are responsible for efficient glucose utilization in E. coli B as determined by microarrays and northern blot analyses. Biotechnol Bioeng 90:805–820
Phue J-N, Shiloach J (2004) Transcription levels of key metabolic genes are the cause for different glucose utilization pathways in E. coli B (BL21) and E. coli K (JM109). J Biotechnol 109:21–30
Pohl S, Marschner K, Storch S, Braulke T (2009) Glycosylation- and phosphorylation-dependent intracellular transport of lysosomal hydrolases. Biol Chem 390:521–527
Poutou-Piñales RA, Vanegas A, Landázuri P, Sáenz H, Lareo L, Echeverri O, Barrera LA (2010) Human sulfatase transiently and functionally active expressed in E. coli K12. Electron J Biotechnol 13. doi:10.2225/vol13-issue3-fulltext-8
Puertas JM, Betton JM (2009) Engineering an efficient secretion of leech carboxypeptidase inhibitor in Escherichia coli. Microb Cell Fact 8:57
Rohrbach M, Clarke J (2007) Treatment of lysosomal storage disorders: progress with enzyme replacement therapy. Drugs 67:2697–2716
Sahdev S, Khattar SK, Saini KS (2008) Production of active eukaryotic proteins through bacterial expression systems: a review of the existing biotechnology strategies. Mol Cell Biochem 307:249–264
Sardiello M, Annunziata I, Roma G, Ballabio A (2005) Sulfatases and sulfatase modifying factors: an exclusive and promiscuous relationship. Hum Mol Genet 14:3203–3217
Shene C, Andrews B, Asenjo J (2003) Study of recombinant micro-organism populations characterized by their plasmid content per cell using a segregated model. Bioprocess Biosyst 25:333–340
Shiloach J, Kaufman J, Guillard A, Fass R (1996) Effect of glucose supply strategy on acetate accumulation, growth, and recombinant protein production by Escherichia coli BL21 (lambdaDE3) and Escherichia coli JM 109. Biotechnol Bioeng 49:421–428
Singh SM, Panda AK (2005) Solubilization and refolding of bacterial inclusion body proteins. J Biosci Bioeng 99:303–310
Swartz J (2001) Advances in Escherichia coli production of therapeutic proteins. Curr Opin Biotechnol 12:195–201
Terpe K (2006) Overview of bacterial expression systems for heterologous protein production: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol 72:211–222
Thomas JG, Baneyx F (1996) Protein misfolding and inclusion body formation in recombinant Escherichia coli cells overexpressing Heat-shock proteins. J Biol Chem 271:11141–11147
Togna A, Shuler M, Wilson D (1993) Effects of plasmid copy number and runaway plasmid replication on overproduction and excretion of beta-lactamase from Escherichia coli. Biotechnol Prog 9:31–39
Tomatsu S, Fukuda M, Masue K, Sukegawa T, Fukao A, Yamagishi T, Hori H, Iwati T, Ogawa Y (1991) Morquio disease: isolation, characterization and expression of full-length cDNA for human N-acetylgalactosamine-6-sulfate sulfatase. Biochem Biophys Res Commun 181:677–683
Tomatsu S, Gutierrez M, Nishioka T, Yamada M, Tosaka Y, Grubb J, Montano A, Vieira M, Trandafirescu G, Pena O, Yamaguchi S, Orii K, Orii T, Noguchi A, Laybauer L (2005) Development of MPS IVA mouse (Galnstm(hC79S.mC76S)slu) tolerant to human N-acetylgalactosamine-6-sulfate sulfatase. Hum Mol Genet 14:3321–3335
Tomatsu S, Montaño A, Gutiérrez M, Grubb J, Oikawa H, Dung V, Ohashi A, Nishioka T, Yamada M, Tosaka Y, Trandafirescu G, Orii T (2007) Characterization and pharmacokinetic study of recombinant human N-acetylgalactosamine-6-sulfate sulfatase. Mol Genet Metab 91:69–78
Tomatsu S, Montaño A, Nishioka T, Gutierrez M, Peña O, Trandafirescu G, Lopez P, Yamaguchi S, Noguchi A, Orri T (2005) Mutation and polymorphism spectrum of the GALNS gene in mucopolysaccharidosis IVA (Morquio A). Hum Mutat 26:500–512
Tomatsu S, Montaño A, Ohashi A, Oikawa H, Oguma T, Dung V, Nishioka T, Orii T, Sly W (2007) Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum Mol Genet 17:815–824
van de Walle M, Shiloach J (1998) Proposed mechanism of acetate accumulation in two recombinant Escherichia coli strains during high density fermentation. Biotechnol Bioeng 57:71–78
van Diggelen O, Zhao H, Kleijer W, Janse H, Poorthuis B, van Pelt J, Kamerling J, Galjaard H (1990) A fluorometric enzyme assay for the diagnosis of Morquio type A. Clin Chem Acta 187:131–140
Vellodi A, Young EP, Cooper A, Wraith JE, Winchester B, Meaney C, Ramaswami U, Will A (1997) Bone marrow transplantation for mucopolysaccharidosis type I: experience of two British centres. Arch Dis Child 76:92–99
Villaverde A, Carrió M (2003) Protein aggregation in recombinant bacteria: biological role of inclusion bodies. Biotechnol Lett 25:1385–1395
Walsh G, Jefferis R (2006) Post-translational modifications in the context of therapeutic proteins. Nat Biotechnol 24:1241–1252
Wang Z, Xiang L, Shao J, Węgrzyn A, Węgrzyn G (2006) Effects of the presence of ColE1 plasmid DNA in Escherichia coli on the host cell metabolism. Microb Cell Fact 5:34. doi:10.1186/1475-2859-1185-1134
Acknowledgments
We thank Dr. Shunji Tomatsu and Dr. Adriana Montaño (Saint Louis University) for pCXN-GALNS plasmid, recombinant human GALNS enzyme, and anti-GALNS monoclonal antibody. We also thank Dr. Patricia Landazuri (Universidad del Quindio) for pGEX-3X expression plasmid, Dr. Raul Poutou (Pontificia Universidad Javeriana) for critical revision of the manuscript, and Carlos Soto for his assistance.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rodríguez, A., Espejo, Á.J., Hernández, A. et al. Enzyme replacement therapy for Morquio A: an active recombinant N-acetylgalactosamine-6-sulfate sulfatase produced in Escherichia coli BL21. J Ind Microbiol Biotechnol 37, 1193–1201 (2010). https://doi.org/10.1007/s10295-010-0766-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0766-x