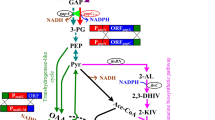
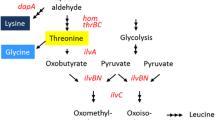
Abstract
Cell growth limitation is known to be an important condition that enhances l-valine synthesis in Corynebacterium glutamicum recombinant strains with l-isoleucine auxotrophy. To identify whether it is the limited availability of l-isoleucine itself or the l-isoleucine limitation-induced rel-dependent ppGpp-mediated stringent response that is essential for the enhancement of l-valine synthesis in growth-limited C. glutamicum cells, we deleted the rel gene, thereby constructing a relaxed (rel − ) C. glutamicum ΔilvA ΔpanB Δrel ilvNM13 (pECKAilvBNC) strain. Variations in enzyme activity and l-valine synthesis in rel + and rel − strains under conditions of l-isoleucine excess and limitation were investigated. A sharp increase in acetohydroxy acid synthase (AHAS) activity, a slight increase in acetohydroxyacid isomeroreductase (AHAIR) activity, and a dramatic increase in l-valine synthesis were observed in both rel + and rel − cells exposed to l-isoleucine limitation. Although the positive effect of induction of the stringent response on AHAS and AHAIR upregulation in cells was not confirmed, we found the stringent response to be beneficial for maintaining increased AHAS, dihydroxyacid dehydratase, and transaminase B activity and l-valine synthesis in cells during the stationary growth phase.



Similar content being viewed by others
References
Arfin SM, Umbarger HE (1969) Purification and properties of the actehydroxy acid isomeroreductase of Salmonella typhimurium. J Biol Chem 244:1118–1127
Blombach B, Schreiner ME, Holátko J, Bartek T, Oldiges M, Eikmanns BJ (2007) l-Valine production with pyruvate dehydrogenase complex-deficient Corynebacterium glutamicum. Appl Environ Microbiol 73:2079–2084. doi:10.1007/s00253-008-1444-z
Blombach B, Schreiner ME, Bartek T, Oldiges M, Eikmanns BJ (2008) Corynebacterium glutamicum tailored for high-yield l-valine production. Appl Microbiol Biotechnol 79:471–479. doi:10.1128/AEM.02826-06
Brockmann-Gretza O, Kalinowski J (2006) Global gene expression during stringent response in Corynebacterium glutamicum in presence and absence of the rel gene encoding (p)ppGpp synthase. BMC Genomics 7:230. doi:10.1186/1471-2164-7-230
Cabiscol E, Tamarit J, Ros J (2000) Oxidative stress in bacteria and protein damage by reactive oxygen species. Int Microbiol 3:3–8
Cioffi EA, Shaw KJ, Bailey WF, Berg CM (1980) Improved synthesis of the sodium salt of dl-α, β-dihydroxyisovaleric acid. Anal Biochem 104:485–488
Cordes C, Mockel B, Eggeling L, Sahm H (1992) Cloning, organization and functional analysis of ilvA, ilvB and ilvC genes from Corynebacterium glutamicum. Gene 112:113–116
Eggeling L (2001) Amino acids. In: Ratledge C, Kristiansen B (eds) Basic biotechnology. Cambridge University Press, London, pp 281–303
Eggeling L, Bott M (2005) Handbook of Corynebacterium glutamicum. CRC Press, Boca Raton
Eggeling I, Cordes C, Eggeling L, Sahm H (1987) Regulation of acetohydroxy acid synthase in Corynebacterium glutamicum during fermentation of α-ketobutyrate to l-isoleucine. Appl Microbiol Biotechnol 25:346–351
Eggeling L, Oberle S, Sahm H (1998) Improved l-lysine yield with Corynebacterium glutamicum: use of dapA resulting in increased flux combined with growth limitation. Appl Microbiol Biotechnol 49:24–30
Elišáková V, Pátek M, Holátko J, Nešvera J, Leyval D, Goergen JL, Delaunay S (2005) Feedback resistant acetoxydroxy acid synthase increases valine production by Corynebacterium glutamicum. Appl Environ Microbiol 71:207–213. doi:10.1128/AEM.71.1.207-213.2005
Eymann C, Homuth G, Scharf C, Hecker M (2002) Bacillus subtilis functional genomics: global characterization of the stringent response by proteome and transcriptome analysis. J Bacteriol 184:2500–2520. doi:10.1128/JB.184.9.2500-2520.2002
Flint DH, Emptage MH, Finnegan MG, Fu W, Johnson MK (1993) The role and properties of the iron-sulfur cluster in Escherichia coli dihydroxy-acid dehydratase. J Biol Chem 268:14732–14742
Hayashi M, Mizoguchi H, Ohnishi J, Mitsubashi H, Yonetani Y, Hashimoto S, Ikeda M (2006) The leuC mutation leading to increased l-lysine production and rel-independent global expression changes in Corynebacterium glutamicum. Appl Microbiol Biotechnol 72:783–789. doi:10.1007/s00253-006-0539-7
Hermann T, Kramer R (1996) Mechanism and regulation of isoleucine excretion in Corynebacterium glutamicum. Appl Environ Microbiol 62:3238–3244
Holátko J, Elišáková V, Prouza M, Sobotka M, Nešvera J, Pátek M (2009) Metabolic engineering of the l-valine biosynthesis pathway in Corynebacterium glutamicum using promoter activity modulation. J Biotechnol 139:203–210. doi:10.1016/j.jbiotec.2008.12.005
Horton RM (1995) PCR-mediated recombination and mutagenesis. SOEing together tailor-made genes. Mol Biotechnol 3:93–99
Jäger W, Schäfer A, Pühler A, Labes G, Wohlleben W (1992) Expression of the Bacillus subtilis sacB gene leads to sucrose sensitivity in the gram-positive bacterium Corynebacterium glutamicum but not in Streptomyces lividans. J Bacteriol 174:5462–5465
Keilhauer C, Eggeling L, Sahm H (1993) Isoleucine synthesis in Corynebacterium glutamicum: molecular analysis of the ilvB-ilvN-ilvC operon. J Bacteriol 175:5595–5603
Kim TH, Kim HJ, Park JS, Kim Y, Kim P, Lee HS (2005) Functional analysis of SigH expression in Corynebacterium glutamicum. Biochem Biophys Res Commun 331:1542–1547. doi:10.1016/j.bbrc.2005.04.073
Kiss RD, Stephanopoulos G (1991) Metabolic activity control of the l-lysine fermentation by restrained growth fed-batch strategies. Biotechnol Prog 7:501–509
Klingenberg M, Pfaff E (1967) Means of terminating reactions. In: Estabroo RW, Pullman ME (eds) Methods in enzymology. Academic Press, New York, pp 680–684
Kuo CF, Mashino T, Fridovich I (1987) a, b-dihydroxyisovalerate dehydratase: a superoxide-sensitive enzyme. J Biol Chem 262:4724–4727
Lange C, Rittman D, Wendisch VF, Bott M, Sahm H (2003) Global expression profiling and physiological characterization of Corynebacterium glutamicum grown in the presence of l-valine. Appl Environ Microbiol 69:2521–2532. doi:10.1128/AEM.69.5.2521-2532.2003
Larisch C, Nakunst D, Huser AT, Tauch A, Kalinowski J (2007) The alternative sigma factor SigB of Corynebacterium glutamicum modulates global gene expression during transition from exponential growth to stationary phase. BMC Genomics 8:4. doi:10.1186/1471-2164-8-4
Leuchtenberger W, Huthmacher K, Drauz K (2005) Biotechnological production of amino acids and derivates: current status and prospects. Appl Microbiol Biotechnol 69:1–8. doi:10.1007/s00253-005-0155-y
Leyval D, Uy D, Delaunay S, Goergen JL, Engasser JM (2003) Characterisation of the enzyme activities involved in the valine biosynthetic pathway in a valine producing strain of Corynebacterium glutamicum. J Biotechnol 104:241–252. doi:10.1016/S0168-1656(03)00162-7
Lowry OH, Rosebrough NJ, Farr LA, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Marienhagen J, Kennerknecht N, Sahm H, Eggeling L (2005) Functional analysis of all aminotransferase proteins inferred from the genome sequence of Corynebacterium glutamicum. J Bacteriol 187:7639–7646. doi:10.1128/JB.187.22.7639-7646.2005
McHardy AC, Tauch A, Ruckert C, Puhler A, Kalinowski J (2003) Genome-based analysis of biosynthetic aminotransferase genes of Corynebacterium glutamicum. J Biotechnol 104:229–240. doi:10.1016/S0168-1656(03)00161-5
Morbach S, Junger C, Sahm H, Eggeling L (2000) Attenuation control of ilvBNC in Corynebacterium glutamicum: evidence of leader peptide formation without the presence of a ribosome binding site. J Biosci Bioeng 90:501–507. doi:10.1016/S1389-1723(01)80030-X
Muniglia L (2001) Elaboration d’une stratégie expérimentale multicritère appliqué à la conception optimale d’un bioprocédé continu de production d’arômes laitiers. PhD thesis. Institut National Polytechnique de Lorraine, Nancy
Nakunst D, Larisch C, Hüser AT, Tauch A, Pühler A, Kalinowski J (2007) The extracytoplasmic function-type sigma factor SigM of Corynebacterium glutamicum ATCC 13032 is involved in transcription of disulfide stress-related genes. J Bacteriol 189:4696–4707. doi:10.1128/JB.00382-07
Patek M, Krumbach K, Eggeling L, Sahm H (1994) Leucine synthesis in Corynebacterium glutamicum: enzyme activities, structure of leuA, and effect of leuA inactivation on lysine synthesis. Appl Environ Microbiol 60:133–140
Radmacher E, Vaitsikova A, Burger U, Krumbach K, Sahm H (2002) Linking central metabolism with increased pathway flux: l–valine accumulation by Corynebacterium glutamicum. Appl Environ Microbiol 68:2246–2250. doi:10.1128/AEM.68.5.2246-2250.2002
Ruklisha M, Shvinka J, Viesturs U (1992) Biotechnology of bacterial synthesis (in Russian, with 1993 annex in English). Zinatne, Riga
Ruklisha M, Damberga B, Shvinka J (1993) Stringent control and ppGpp synthesis in Brevibacterium flavum during amino acid starvation. Proceed Acad Sci Latv 12:59–62
Ruklisha M, Viesturs U, Labane L (1995) Growth control and ppGpp synthesis in Brevibacterium flavum cells at various medium mixing rates and aeration intensities. Acta Biotechnol 15:41–48
Ruklisha M, Ionina R, Paegle L, Petrovica G (2001) Metabolism and lysine biosynthesis control in Brevibacterium flavum: impact of stringent response. In: Simon JP, Durieux A (eds) Applied microbiology, vol 2. Kluwer, Dordrecht, pp 51–57
Ruklisha M, Paegle L, Denina I (2007) l-valine biosynthesis during batch and fed-batch cultivations of Corynebacterium glutamicum: relationship between changes in bacterial growth rate and intracellular metabolism. Proc Biochem 42:634–640. doi:10.1016/j.procbio.2006.11.008
Sahm H, Eggeling L (1999) D-pantothenate synthesis in Corynebacterium glutamicum and use of panBC and genes encoding l-valine synthesis for d-pantothenate overproduction. Appl Environ Microbiol 65:1973–1979
Shvinka JE, Toma MK, Galinina NI, Skards IV, Viesturs UE (1979) Production of superoxide radicals during bacterial respiration. J Gen Microbiol 113:377–382
Shvinka J, Viesturs U, Ruklisha M (1980) Yield regulation of lysine biosynthesis in Brevibacterium flavum. Biotechnol Bioeng 22:897–912
Tauch A, Wehmeier L, Gotker S, Puhler A, Kalinowski J (2001) Relaxed rrn expression and amino acid requirement of a Corynebacterium glutamicum rel mutant defective in (p)ppGpp metabolism. FEMS Microbiol Lett 201:53–58
Tedin K, Norel F (2001) Comparison of ΔrelA strains of Escherichia coli and Salmonella enterica serovar typhimurium suggests a role for ppGpp in attenuation regulation of branched-chain amino acid biosynthesis. J Bacteriol 83:6184–6196. doi:10.1128/JB.183.21.6184-6196.2001
Wehmeier L, Schafer A, Burkovski A, Kramer R, Mechold U, Malke H, Puhler A, Kalinowski J (1998) The role of the Corynebacterium glutamicum rel gene in (p)ppGpp metabolism. Microbiology 144:1853–1862
Westerfeld WW (1945) A colorimetric determination of blood acetoin. J Biol Chem 161:495–502
Zhang X, Dennis P, Ehrenberg M, Bremer H (2002) Kinetic properties of rrn promoters in Escherichia coli. Biochimie 84:981–996. doi:10.1016/S0300-9084(02)00010-X
Acknowledgments
This work was supported by grant No. 04.1112 of the Research Council of the Academy of Sciences of the Republic of Latvia and by grant No. 204/07/J012 from the Scientific Council of the Czech Republic. I. Denina was supported by a grant from the European Social Fund (ESF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Denina, I., Paegle, L., Prouza, M. et al. Factors enhancing l-valine production by the growth-limited l-isoleucine auxotrophic strain Corynebacterium glutamicum ΔilvA ΔpanB ilvNM13 (pECKAilvBNC). J Ind Microbiol Biotechnol 37, 689–699 (2010). https://doi.org/10.1007/s10295-010-0712-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0712-y