Abstract
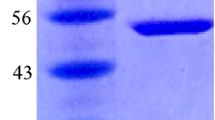
A recombinant Thermotoga maritima β-glucosidase A (BglA) was purified to homogeneity for performing enzymatic hydrolysis of isoflavone glycosides from soy flour. The kinetic properties K m, k cat, and k cat/K m of BglA towards isoflavone glycosides, determined using high-performance liquid chromatography, confirmed the higher efficiency of BglA in hydrolyzing malonylglycosides than non-conjugated glycosides (daidzin and genistin). During hydrolysis of soy flour by BglA at 80°C, the isoflavone glycosides (soluble form) were extracted from soy flour (solid state) into the solution (liquid state) in thermal condition and converted to their aglycones (insoluble form), which mostly existed in the pellet to be separated from BglA in the reaction solution. The enzymatic hydrolysis in one-step and two-step approaches yielded 0.38 and 0.35 mg genistein and daidzein per gram of soy flour, respectively. The optimum conditions for conversion of isoflavone aglycones were 100 U per gram of soy flour, substrate concentration 25% (w/v), and incubation time 3 h for 80°C.




Similar content being viewed by others
References
Kaya M, Ito J, Kotaka A, Matsumura K, Bando H, Sahara H, Ogino C, Shibasaki S, Kuroda K, Ueda M, Kondo A, Hata Y (2008) Isoflavone aglycones production by display of β-glucosidase from on yeast cell surface. Appl Microbiol Biotechnol 79:51–60
Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M (2000) Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr 130:1695–1699
Kawakami Y, Tsurugasaki W, Nakamura S, Osada K (2005) Comparison of regulative functions between dietary soy isoflavones aglycone and glucoside on lipid metabolism in rats fed cholesterol. J Nutr Biochem 16:205–212
Xu X, Hariss KS, Wang HJ, Murphy PA, Hendrich S (1995) Bioavailability of soy isoflavones depends upon gut microflora in women. J Nutr 125:2307–2315
Brown KD, Brown NM, Zimmer-Nechemias L, Brashear WT, Wolfe BE, Kirschner AS, Heubi JE (2002) Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am J Clin Nutr 76:447–453
Kao TH, Chien JT, Chen BH (2008) Extraction yield of isoflavones from soy cake as affected by solvent and supercritical carbon dioxide. Food Chem 107:1728–1736
Kuo LC, Cheng WY, Wu RY, Huang CJ, Lee KT (2006) Hydrolysis of black soy isoflavone glycosides by Bacillus subtilis natto. Appl Microbiol Biotechnol 73:314–320
Chuankhayan P, Rimlumduan T, Svasti J, Cairns JRK (2007) Hydrolysis of soy isoflavonoid glycosides by Dalbergia β-glucosidases. J Agric Food Chem 55:2407–2412
Eisen B, Ungar Y, Shimoni E (2003) Stability of isoflavones in soy milk stored at elevated and ambient temperature. J Agric Food Chem 51:2212–2215
Gabelsberger J, Liebl W, Schleifer KH (1993) Purification and properties of recombinant β-glucosidase of the hyperthermophilic bacterium Thermotoga maritima. Appl Microbiol Biotechnol 40:44–52
Xue YM, Shao WL (2004) Expression and characterization of a thermostable β-xylosidase from hyperthermophile Thermotoga maritima. Biotechnol Lett 26:1511–1515
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Briante R, Cara FL, Febbraio F, Barone R, Piccialli G, Carolla R, Mainolfi P, Napoli LD, Patumi M, Fontanazza G, Nucci R (2000) Hydrolysis of oleuropein by recombinant β-glycosidase from hyperthermophilic archaeon Sulfolobus solfataricus immobilised on chitosan matrix. J Biotechnol 77:275–286
Suzuki H, Watanabe R, Fukushima Y, Fujita N, Noguchi A, Yokoyama R, Nishitani K, Nishino T, Nakayama T (2006) An isoflavone conjugate-hydrolyzing β-glucosidase from the roots of soy (Glycine max) seedlings. J Biol Chem 281(40):30251–30259
Mathias K, Baraem I, Carlos MC, Kirby DH (2006) Heat and pH effects on the conjugated forms of genistin and daidzin isoflavones. J Agric Food Chem 54:7495–7502
Jiang DH, Tian JJ, Song HZ, Bai ZM (2008) Comparison between hydrolysis methods of soy isoflavones. Cereal Food Ind 15(1):25–27
Ravindranath MH, Muthugounder S, Presser N, Viswanathan S (2004) Anticancer therapeutic potential of soy isoflavone, genistein. Adv Exp Med Biol 546:121–165
Lamartiniere CA (2000) Protection against breast cancer with genistein: a component of soy. Am J Clin Nutr 71(6):1705–1707
Weaver CM, Cheong JM (2005) Soy isoflavones and bone health: the relationship is still unclear. J Nutr 135:1243–1247
Cotter A, Cashman KD (2003) Genistein appears to prevent early postmenopausal bone loss as effectively as hormone replacement therapy. Nutr Rev 61:346–351
Acknowledgments
Thanks to Prof. Shao, W.L., for the suggestions for this research. This work was supported by NSF of Jiangsu Province of China (project BK2006220), by NSF of the Higher School of Jiangsu Province of China (project 05KJB180059), and by a grant from Nanjing Normal University (04104XGQ2B59).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xue, Y., Yu, J. & Song, X. Hydrolysis of soy isoflavone glycosides by recombinant β-glucosidase from hyperthermophile Thermotoga maritima . J Ind Microbiol Biotechnol 36, 1401–1408 (2009). https://doi.org/10.1007/s10295-009-0626-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-009-0626-8