Abstract
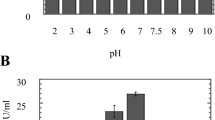
Myrothecium verrucaria is a nondermatophytic filamentous fungus able to grow and to produce keratinase in submerged (93.0 ± 19 U/ml) and solid state (98.8 ± 7.9 U/ml) cultures in which poultry feather powder (PFP) is the only substrate. The purpose of the present work was to verify how different carbon and nitrogen sources can influence the production of keratinase by this fungus. Addition of carbohydrates, such as glucose and sucrose, caused only slight improvements in keratinase production, but the addition of starch caused a significant improvement (135.0 ± 25 U/ml). The highest levels of keratinase activity, however, were obtained by supplementing the PFP cultures with cassava bagasse, 168.0 ± 28 U/ml and 189.0 ± 26 U/ml in submerged and solid state cultures, respectively. Contrarily, the supplementation of PFP medium with organic or inorganic nitrogen sources, such as casein, soy bean protein, gelatin, ammonium nitrate and alanine, decreased the production of keratinase in both types of cultures (around 20 U/ml), showing that the production of keratinase by M. verrucaria is repressed by nitrogen sources. The results obtained in this work suggest that the association of the two residues PFP plus cassava bagasse could be an excellent option as a cheap culture medium for the production of keratinase in submerged and solid state cultures.





Similar content being viewed by others
References
Anbu P, Gobinath SCB, Hilda A, Lakshmipriya T, Annadurai G (2007) Optimization of extracellular keratinase by poultry farm isolate Scopulariopis brevicaulis. Bioresour Technol 98:1298–1303. doi:10.1016/j.biortech.2006.05.047
Anbu P, Hilda A, Sur H-W, Hur B-K, Jayanthi S (2008) Extracellular keratinase from Trichophyton sp HA-2 isolated from feather dumping soil. Int Biodeter Biodegrad 62:287–292. doi:10.1016/j.ibiod.2007.07.017
Bockle B, Müller R (1997) Reduction of disulfide bonds by Streptomyces pactum during growth on chicken feathers. Appl Environ Microbiol 63:790–792
Boyette CD, Hoagland RE, Abbas HK (2007) Evaluation of the bioherbicide Myrothecium verrucaria for weed control in tomato (Lycopersicon esculentum). Biocontrol Sci Technol 17:171–178. doi:10.1080/09583150600937451
Brandelli A (2008) Bacterial keratinases: useful enzymes for bioprocessing agro-industrial wastes and beyond. Food Bioprocess Technol 1:105–116. doi:10.1007/s11947-007-0025-y
Dalev P, Ivanov I, Liubomirova A (1997) Enzymic modification of feather keratin hydrolyzates with lysine aimed at increasing the biological value. J Sci Food Agric 73:242–244. doi:10.1002/(SICI)1097-0010(199702)73:2<242::AID-JSFA712>3.0.CO;2-3
De Azeredo LAI, Lima MB, de-Coleho RRR, Freire DMG (2006) Thermophilic protease production by Streptomyces sp. 594 in submerged and solid state fermentation using feather meal. J Appl Microbiol 100:641–647. doi:10.1111/j.1365-2672.2005.02791.x
El-Naghy MA, El-Ktatny MS, Fadl-Allah EM, Nazeer WW (1998) Degradation of chicken feathers by Chrysosporium georgiae. Mycopathologia 143:77–84. doi:10.1023/A:1006953910743
Esawy MA (2007) Isolation and partial characterization of extracellular keratinase from a novel mesophilic Streptomyces albus AZA. Res J Agric Biol Sci 3:808–817
Farag AM, Hassan MA (2004) Purification, characterization and immobilization of a keratinase from Aspergillus oryzae. Enzyme Microb Technol 34:85–93. doi:10.1016/j.enzmictec.2003.09.002
Frieddrich AB, Antranikian G (1996) Keratin degradation by Fervidobacterium pennavorans, a novel thermophilic anaerobic species of the order thermotogales. Appl Environ Microbiol 62:2875–2882
Gassesse A, Kaul RH, Gashe BA, Mattiasson B (2003) Novel alkaline proteases from alkalophilic bacteria grown on chicken feather. Enzyme Microb Technol 32:519–524. doi:10.1016/S0141-0229(02)00324-1
Ghosh A, Chakrabarti K, Chattopadhyay D (2008) Degradation of raw feather by a novel high molecular weight extracellular protease from newly isolated Bacillus cereus DCUW. J Ind Microbiol Biotechnol 35:825–834. doi:10.1007/s10295-008-0354-5
Gradisar H, Kern S, Frieddrich J (2000) Keratinase of Doratomyces microsporus. Appl Microbiol Biotechnol 53:196–200. doi:10.1007/s002530050008
Gradisar H, Friedrich J, Krizaj I, Jerala R (2005) Similarities and specificities of fungal keratinolytic proteases: comparison of keratinases of Paecilomyces marquandii and Doratomyces microsporus to some known proteases. Appl Environ Microbiol 71:3420–3426. doi:10.1128/AEM.71.7.3420-3426.2005
Gupta R, Ramnani P (2006) Microbial keratinases and their prospective applications: an overview. Appl Microbiol Biotechnol 70:21–33. doi:10.1007/s00253-005-0239-8
Kaul S, Sambali G (1999) Production of extracellular keratinases by keratinophilic fungal species inhabiting feathers of living poultry birds (Gallus domesticus): a comparison. Mycopathologia 146:19–24. doi:10.1023/A:1007086720237
Kunert J, Truper HG (1986) Cystine catabolism in mycelia of Microsporum gypseum, a dermatophytic fungus. Arch Microbiol 150:600–601. doi:10.1007/BF00408257
Malviya HK, Rajak RC, Hasija SK (1992) Synthesis and regulation of extracellular keratinase in three fungi isolated from the grounds of a gelatin factory, Jabalpur, India. Mycopathologia 120:1–4. doi:10.1007/BF00578494
Manczinger L, Rozs M, Vagvolgyi CS, Kevei F (2003) Isolation and characterization of a new keratinolytic Bacillus licheniformis strain. World J Microbiol Biotechnol 19:35–39. doi:10.1023/A:1022576826372
Marcondes NR, Taira CL, Vandresen DC, Svidzinski TIE, Kadowaki MK, Peralta RM (2007) New-feather-degrading filamentous fungi. Microb Ecol 55:1–5
Mitchell DA, Berovic N, Krieger K (2002) Overview of solid state bioprocessing. Biotechnol Annu Rev 8:183–225. doi:10.1016/S1387-2656(02)08009-2
Moreira FG, Souza CGM, Costa MAF, Reis S, Peralta RM (2007) Degradation of keratinous materials by the plant pathogenic fungus Myrothecium verrucaria. Mycopathologia 163:153–160. doi:10.1007/s11046-007-0096-3
Moritz JS, Latshaw JD (2001) Indicators of nutritional value of hydrolyzed feather meal. Poult Sci 80:79–86
Nam GW, Lee DW, Lee NJ, Kim BC, Choe EA, Hwang JK, Suhartono MT, Pyun YR (2002) Native feather degradation by Fervidobacterium islandicum AW–1, a newly isolated keratinase-producing thermophilic anaerobe. Arch Microbiol 178:538–547. doi:10.1007/s00203-002-0489-0
Nickerson WJ, Noval JJ, Robinson RS (1963) Keratinase I. Properties of the enzyme conjugate elaborated by Streptomyces fradie. Biochim Biophys Acta 77:73–86. doi:10.1016/0006-3002(63)90470-0
Odetallah NH, Wang JJ, Garlich JD, Shih JCH (2003) Keratinase in starter diets improves growth of broiler chicks. Poult Sci 82:664–670
Okafor JI, Ada N (2000) Keratinolytic activity of five human isolates of the dermatophytes. J Commun Dis 32:300–305
Onifade AA, Al-Sane NA, Al-Musallam AA, Al-Zarban S (1998) A Review: potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and others keratins as livestock feed resources. Bioresour Technol 66:1–11. doi:10.1016/S0960-8524(98)00033-9
Pandey A, Soccoll CR, Nigam P, Soccoll VT, Vandenberghe LPS, Mohan R (2000) Biotechnological potential of agroindustrial residues. II. Cassava bagasse. Bioresour Technol 74:81–87. doi:10.1016/S0960-8524(99)00143-1
Rissen S, Antranikian G (2001) Isolation of Thermoanaerobacter keratinophilus sp nov., a novel thermophilic, anaerobic bacterium with keratinolytic activity. Extremophiles 5:399–408. doi:10.1007/s007920100209
Sangali S, Brandelli A (2000) Feather keratin hydrolysis by a Vibrio sp strain kr2. J Appl Microbiol 89:735–743. doi:10.1046/j.1365-2672.2000.01173.x
Santos RMDB, Firmino ALP, de Sá CM, Felix CR (1996) Keratinolytic activity of Aspergillus fumigatus Fresenius. Curr Microbiol 33:364–370. doi:10.1007/s002849900129
Singh CJ (1997) Characterization of an extracellular keratinase of Trichophyton simii and its role in keratin degradation. Mycopathologia 137:13–16. doi:10.1023/A:1006844201399
Singh CJ (1999) Exocellular proteases of Malbranchea gypsea and their role in keratin deterioration. Mycopathologia 143:147–150. doi:10.1023/A:1006968600404
Son H-J, Park H-C, Kim H-S, Lee C-Y (2008) Nutritional regulation of keratinolytic activity in Bacillus pumilis. Biotechnol Lett 30:461–465. doi:10.1007/s10529-007-9567-3
Vianni FC, dos Santos JI, Paula CR, Larson CE, Gambale W (2001) Production of extracellular enzymes by Microsporum canis and their role in its virulence. Med Mycol 39:463–468. doi:10.1080/714031047
Yamamura S, Morita Y, Hasan Q, Yokoyama K, Tamiya E (2002) Keratin degradation: a cooperative action of two enzymes from Stenotrophomonas sp. Biochem Biophys Res Commun 294:1138–1143. doi:10.1016/S0006-291X(02)00580-6
Acknowledgments
This study was supported by grants from Conselho Nacional de Pesquisa e Desenvolvimento (CNPq) and Fundação Araucária. RM Peralta is research fellow of CNPq. FG Moreira-Guillen is a recipient of CAPES Fellowships. We thank A Chaves and MAF Costa for their technical assistances.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Gioppo, N.M.R., Moreira-Gasparin, F.G., Costa, A.M. et al. Influence of the carbon and nitrogen sources on keratinase production by Myrothecium verrucaria in submerged and solid state cultures. J Ind Microbiol Biotechnol 36, 705–711 (2009). https://doi.org/10.1007/s10295-009-0540-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-009-0540-0