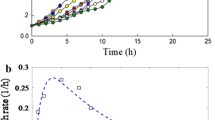
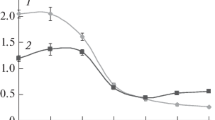
Abstract
Cyanuric acid (1,3,5-triazine-2,4,6-triol [OOOT]) is a common biodegradation byproduct of triazinic herbicides, frequently accumulated in soils or water when supplementary carbon sources are absent. A binary bacterial culture able to degrade OOOT was selected through a continuous selection process accomplished in a chemostat fed with a mineral salt (MS) medium containing cyanuric acid as the sole carbon and nitrogen source. By sequence comparison of their 16S rDNA amplicons, bacterial strains were identified as Agrobacterium tumefaciens, and Acinetobacter sp. When the binary culture immobilized in a packed bed reactor (PBR) was fed with MS medium containing OOOT (50 mg L−1), its removal efficiencies were about 95%; when it was fed with OOOT plus glucose (120 mg L−1) as a supplementary carbon source, its removal efficiencies were closer to 100%. From sessile cells, attached to PBR porous support, or free cells present in the outflowing medium, DNA was extracted and used for Random Amplification of Polymorphic DNA analysis. Electrophoretic patterns obtained were compared to those of pure bacterial strains, a clear predominance of A. tumefaciens in PBR was observed. Although in continuous suspended cell culture, a stable binary community could be maintained, the attachment capability of A. tumefaciens represented a selective advantage over Acinetobacter sp. in the biofilm reactor, favoring its predominance in the porous stone support.






Similar content being viewed by others
References
Sullivan KB, Spence KM (2003) Effect of sublethal concentration of atrazine and nitrate on metamorphosis of the Africa clawed frog. Environ Toxicol Chem 22:627–635. doi:10.1897/1551-5028(2003)022<0627:EOSCOA>2.0.CO;2
Gammon DW, Aoldous CN, Carr WC, Sanborn JN, Pfeifer KF (2005) A risk assessment of atrazine use in California: human health and ecological aspects. Pest Manag Sci 61:331–355. doi:10.1002/ps.1000
Office of Pesticide Programs (2000) Pesticide ecotoxicity database. Environmental fate and effects division. USEPA, Washington, DC
Loeb HA, Kelly WH (1963) Acute oral toxicity of 1,496 chemicals force-fed to carp. Spec. Sci. Rep. Fish. No. 471. Fish Wildl. Serv. U.S.D.I, Washington, DC, p 124
Applegate VC, Howell JH, Hall AE Jr, Smith MA (1957) Toxicity of 4,346 chemicals to larval lampreys and fishes. Spec. Sci. Rep. Fish. No. 207. Fish Wildl. Serv. U.S.D.I, Washington, DC, p 157
Magnuson ML, Kelty CA, Cantú R (2001) Stable association complex electrospray mass spectrometry for the determination of cyanuric acid. J Am Soc Mass Spectrom 12:1085–1091. doi:10.1016/S1044-0305(01)00292-6
Panderi I (2003) Porous graphitized carbon columns in liquid chromatography. In: Cazes J (ed) The encyclopedia of chromatography. Marcel Dekker, Inc, New York Electronic Version
Shapir N, Mongodin EF, Sadowsky MJ, Daugherty SC, Nelson KE, Wackett LP (2007) Evolution of catabolic pathways: genomic insights into microbial s-triazine metabolism. J Bacteriol 189:674–682. doi:10.1128/JB.01257-06
Sisodia SS, Weber AS, Jensen JN (1996) Continuous culture biodegradation of simazine’s chemical oxidation products. Water Res 30:2055–2064. doi:10.1016/0043-1354(96)00099-1
Chan CY, Tao S, Dawson R, Wong PK (2004) Treatment of atrazine by integrating photocatalytic and biological processes. Environ Pollut 131:45–54. doi:10.1016/j.envpol.2004.02.022
Watanabe N, Horikoshi N, Hidaka H, Serpone N (2005) On the recalcitrant nature of the triazinic ring species, cyanuric acid, to degradation in Fenton solution and in UV-illuminated TiO2 (naked) and fluorinated TiO2 aqueous dispersions. J Photochem Photobiol Chem 174:229–238. doi:10.1016/j.jphotochem.2005.03.013
Rodríguez EM, Álvarez PM, Rivas FJ, Beltrán FJ (2004) Wet peroxide degradation of atrazine. Chemosphere 54:71–78. doi:10.1016/S0045-6535(03)00701-X
Parra S, Stanca SE, Guasaquillo I, Thampi KR (2004) Photocatalytic degradation of atrazine using suspended and supported TiO2. Appl Catal B Environ 51:107–116. doi:10.1016/j.apcatb.2004.01.021
Johnson DC, Feng J, Houk LL (2000) Direct electrochemical degradation of organic wastes in aqueous media. Electrochim Acta 46:323–330. doi:10.1016/S0013-4686(00)00588-0
Horikoshi S, Hidaka H (2003) Non-degradable triazine substrates of atrazine and cyanuric acid hydrothermally and in supercritical water under the UV-illuminated photocatalytic cooperation. Chemosphere 51:139–142. doi:10.1016/S0045-6535(02)00786-5
Farré MJ, Franch MI, Malato S, Ayllón JA, Peral J, Doménech X (2005) Degradation of some biorecalcitrant pesticides by homogeneous and heterogeneous photocatalytic ozonation. Chemosphere 58:1127–1133. doi:10.1016/j.chemosphere.2004.09.064
Ikehata K, El-Din MG (2006) Aqueous pesticide degradation by hydrogen peroxide/ultraviolet irradiation and Fenton-type advanced oxidation processes: a review. J Environ Eng Sci 5:81–135. doi:10.1139/s05-046
Jesse JA, Benoit RE, Hendricks AC, Allen GC, Neal JL (1983) Anaerobic degradation of cyanuric acid, cysteine and atrazine by a facultative anaerobic bacterium. Appl Environ Microbiol 45:97–102
Gonzalez-Barreiro O, Rioboo C, Herrero C, Cid A (2006) Removal of triazine herbicides from freshwater systems using photosynthetic microorganisms. Environ Pollut 144:266–271. doi:10.1016/j.envpol.2005.12.014
Feakin SJ, Blackburn E, Burns RG (1995) Inoculation of granular activated carbon in a fixed bed with S-triazine-degrading bacteria as a water treatment process. Water Res 29:819–825. doi:10.1016/0043-1354(94)00209-P
Saez F, Pozo C, Gomez MA, Martinez-Toledo MV, Rodelas B, Gonzalez-Lopez J (2006) Growth and denitrifying activity of Xanthobacter autotrophicus CECT 7064 in the presence of selected pesticides. Appl Microbiol Biotechnol 71:563–567. doi:10.1007/s00253-005-0182-8
Nishimura K, Yamamoto M, Nakagomi T, Takiguchi Y, Naganuma T, Uzuka Y (2002) Biodegradation of triazine herbicides on polyvinylalcohol gel plates by the soil yeast Lipomyces starkeyi. Appl Microbiol Biotechnol 58:848–852. doi:10.1007/s00253-002-0950-7
Fragoeiro S, Magan S (2005) Enzymatic activity, osmotic stress and degradation of pesticide mixtures in soil extract liquid broth inoculated with Phanerochaete chrysosporium and Trametes versicolor. Environ Microbiol 7:348–355. doi:10.1111/j.1462-2920.2005.00699.x
Grigg BC, Bischoff M, Turco RF (1997) Cocontaminant effects on degradation of triazine herbicides by a mixed microbial culture. J Agric Food Chem 45:995–1000. doi:10.1021/jf9604910
Saldick J (1974) Biological treatment of plant waste streams to remove cyanuric acid. US Patent 3,926,795
Zeyer J, Hutter R, Mayer P (1981) Process for the degradation of cyanuric acid. US Patent 4,274,955
Bagnall EA, Gurvitch MM, Horner RL (1984) Biological decomposition of cyanuric acid. UK Patent GB 2,127,006 A
Cook AM Hütter R. (1988) Process for the degradation of s-triazine derivatives in aqueous solutions. US Patent 4,745,064
Osadchaia AI, Kudriavtsev VA, Safronova LA, Smirnov VV (1999) The effect of the nutritional sources on the synthesis of exopolysaccharides and amino acids by Bacillus subtilis strains. Mikrobiol Z 61:56–63
Atkinson B, Mavituna F (1983) Biochemical engineering and biotechnology handbook. Macmillan Publishers Ltd, Surrey
Relman DA (1993) Universal bacterial 16S rDNA amplification and sequencing. In: Persing HD, Smith FT, Tenover CF, White JT (eds) Diagnostic molecular microbiology. Principles and applications. AMS, Washington, DC
Felske A, Engelen B, Nübel U, Backhaus H (1996) Direct ribosome isolation from soil to extract bacterial rRNA for community analysis. Appl Environ Microbiol 62:4162–4167
Renders N, van Belkum A, Barth A, Goessens W, Mouton J, Verbrugh H (1996) Typing of Pseudomonas aeruginosa strains from patients with cystic fibrosis: phenotyping versus genotyping. Clin Microbiol Infect 1:261–265
Hodge DS, Devinny JS (1995) Modeling removal of air contaminants by biofiltration. J Environ Eng 121:21–32. doi:10.1061/(ASCE)0733-9372(1995)121:1(21)
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin–phenol reagents. J Biol Chem 193:265–275
Karns JS (1999) Gene sequence and properties of an s-triazine ring-cleavage enzyme from Pseudomonas sp. strain NRRLB-12227. Appl Environ Microbiol 65:3512–3517
Strong CL, Rosendahl C, Johnson G, Sadowsky JM, Wacket LP (2002) Arthrobacter aurescens TC1 metabolizes diverse s-triazine ring compounds. Appl Environ Microbiol 68:5973–5980. doi:10.1128/AEM.68.12.5973-5980.2002
HACH-Wastewater and biosolids analysis manual (1999) HACH Company, USA
Fruchey I, Shapir N, Sadowsky MJ, Wackett LP (2003) On the origins of cyanuric acid hydrolase: purification, substrates and prevalence of AtzD from Pseudomonas sp. strain ADP. Appl Environ Microbiol 69:3653–3657. doi:10.1128/AEM.69.6.3653-3657.2003
Kodama T, Ding L, Yoshida M, Yajima M (2001) Biodegradation of an s-triazine herbicide, simazine. J Mol Catal B Enzym 11:1073–1078. doi:10.1016/S1381-1177(00)00169-7
Breedveld MW, Miller K (1994) Cyclic glucans of members of the family Rhizobiaceae. Microbiol Rev 58:145–161
Williamson G, Damani K, Devenney P, Faulds CB, Morris VJ, Stevens BJH (1992) Mechanism of action of cyclic ß(1–2)-glucan synthetase from Agrobacterium tumefaciens: competition between cyclization and elongation reactions. J Bacteriol 174:7941–7947
Baumann P, Duodoroff M, Stanier RY (1968) A study of Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol 95:1520–1541
Bergogne-Bérézin E, Towner KJ (1996) Acinetobacter spp. as nosocomial pathogens: microbiological, clinical and epidemiological features. Clin Microbiol Rev 9:148–165
Gohl O, Friedrich A, Hoppert M, Averhoff B (2006) The thin pili of Acinetobacter sp. strain BD413 mediate adhesion to biotic and abiotic surfaces. Appl Environ Microbiol 72:1394–1401. doi:10.1128/AEM.72.2.1394-1401.2006
An D, Danhorn T, Fuqua C, Parsek MR (2006) Quorum sensing and motility mediate interactions between Pseudomonas aeruginosa and Agrobacterium tumefaciens in biofilm cocultures. Proc Natl Acad Sci USA 103:3828–3833. doi:10.1073/pnas.0511323103
Huang C-T, Peretti SW, Bryers JD (1994) Effects of medium carbon-to-nitrogen ratio on biofilm formation and plasmid stability. Biotechnol Bioeng 44:329–336. doi:10.1002/bit.260440310
Thompson LJ, Gray V, Lindsay D, von Holy A (2006) Carbon: nitrogen:phosphorus ratios influence biofilm formation by Enterobacter cloacae and Citrobacter freundii. J Appl Microbiol 101:1105–1113. doi:10.1111/j.1365-2672.2006.03003.x
Merritt PM, Danhorn T, Fuqua C (2007) Motility ans chemotaxis in Agrobacterium tumefaciens surface attachment and biofilm formation. J Bacteriol 189:8005–8014. doi:10.1128/JB.00566-07
Fuqua C (2008) Agrobacterium–host attachment and biofilm formation. In: Tzira T, Citovsky V (eds) Agrobacterium: from biology to biotechnology. Springer, New York
Jensen HL, Abdel-Ghaffer AS (1969) Cyanuric acid as nitrogen source for microorganisms. Arch Mikrobiol 67:1–5. doi:10.1007/BF00413674
Wolf DC, Martin JP (1975) Microbial decomposition of Ring-14C Atrazine, cyanuric acid, and 2-Chloro-4, 6-diamino-s-triazine. J Environ Qual 4:134–139
Shiomi N, Yamaguchi Y, Nakai H, Fujita T, Katsuda T, Katoh S (2006) Degradation of cyanuric acid in soil by Pseudomonas sp. NRRL B-12227 using bioremediation with self-immobilization system. J Biosci Bioeng 102(3):206–209. doi:10.1263/jbb.102.206
Acknowledgments
S.P. Galíndez-Nájera and M.A. Llamas-Martínez, are holders of a research grant from PIFI-IPN. C. Juárez-Ramírez, N. Ruiz-Ordaz, D. Ahuatzi-Chacón, and J. Galíndez-Mayer, are holders of grants from COFAA-IPN, SIP-IPN, and SNI-Conacyt.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galíndez-Nájera, S.P., Llamas-Martínez, M.A., Ruiz-Ordaz, N. et al. Cyanuric acid biodegradation by a mixed bacterial culture of Agrobacterium tumefaciens and Acinetobacter sp. in a packed bed biofilm reactor. J Ind Microbiol Biotechnol 36, 275–284 (2009). https://doi.org/10.1007/s10295-008-0496-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0496-5