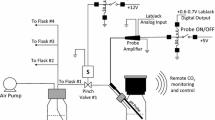
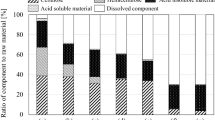
Abstract
A 2 M sodium acetate buffer at pH 4.2 was tried to simplify the step of pH adjustment in a laboratory dry-grind procedure. Ethanol yields or conversion efficiencies of 18 sorghum hybrids improved significantly with 2.0–5.9% (3.9% on average) of relative increases when the method of pH adjustment changed from traditional HCl to the acetate buffer. Ethanol yields obtained using the two methods were highly correlated (R 2 = 0.96, P < 0.0001), indicating that the acetate buffer did not influence resolution of the procedure to differentiate sorghum hybrids varying in fermentation quality. Acetate retarded the growth of Saccharomyces cerevisiae, but did not affect the overall fermentation rate. With 41–47 mM of undissociated acetic acid in mash of a sorghum hybrid at pH 4.7, rates of glucose consumption and ethanol production were inhibited during exponential phase but promoted during stationary phase. The maximum growth rate constants (μ max) were 0.42 and 0.32 h−1 for cells grown in mashes with pH adjusted by HCl and the acetate buffer, respectively. Viable cell counts of yeast in mashes with pH adjusted by the acetate buffer were 36% lower than those in mashes adjusted by HCl during stationary phase. Coupled to a 5.3% relative increase in ethanol, a 43.6% relative decrease in glycerol was observed, when the acetate buffer was substituted for HCl. Acetate helped to transfer glucose to ethanol more efficiently. The strain tested did not use acetic acid as carbon source. It was suggested that decreased levels of ATP under acetate stress stimulate glycolysis to ethanol formation, increasing its yield at the expense of biomass and glycerol production.






Similar content being viewed by others
References
AACC International (2000) Approved methods of the American association of cereal chemists, 10th edn. Methods 44-15A and 76-13. AACC International, St Paul
Abbott DA, Ingledew WM (2004) Buffering capacity of whole corn mash alters concentrations of organic acids required to inhibit growth of Saccharomyces cerevisiae and ethanol production. Biotechnol Lett 26:1313–1316. doi:10.1023/B:BILE.0000044924.76429.71
Bideaux C, Alfenore S, Cameleyre X, Molina-Jouve C, Uribelarrea JL, Guillouet SE (2006) Minimization of glycerol production during the high-performance fed-batch ethanolic fermentation process in Saccharomyces cerevisiae, using a metabolic model as a prediction tool. Appl Environ Microbiol 72:2134–2140. doi:10.1128/AEM.72.3.2134-2140.2006
Cronwright GR, Rohwer JM, Prior BA (2002) Metabolic control analysis of glycerol synthesis in Saccharomyces cerevisiae. Appl Environ Microbiol 68:4448–4456. doi:10.1128/AEM.68.9.4448-4456.2002
Devantier R, Pedersen S, Olsson L (2005) Characterization of very high gravity ethanol fermentation of corn mash. Effect of glucoamylase dosage, pre-saccharification and yeast strain. Appl Microbiol Biotechnol 68:622–629. doi:10.1007/s00253-005-1902-9
Domberk KM, Ingram LO (1987) Ethanol production during batch fermentation with Saccharomyces cerevisiae: changes in glycolytic enzymes and internal pH. Appl Environ Microbiol 53:1286–1291
Freese E, Sheu CW, Galliers E (1973) Function of lipophilic acids as antimicrobial food additives. Nature 24:321–325. doi:10.1038/241321a0
Imai T, Ohno T (1995) Measurement of yeast intracellular pH by image processing and the change it undergoes during growth phase. J Biotechnol 38:165–172. doi:10.1016/0168-1656(94)00130-5
Imai T, Ohno T (1995) The relationship between viability and intracellular pH in the yeast Saccharomyces cerevisiae. Appl Environ Microbiol 61:3604–3608
Ingledew WM, Thomas KC, Hynes SH, McLeod JG (1999) Viscosity concerns with rye mashes used for ethanol production. Cereal Chem 76:459–464. doi:10.1094/CCHEM.1999.76.3.459
Ingledew WM, Jones AM, Bhatty RS, Rossnagel BG (1995) Fuel alcohol production from hull-less barley. Cereal Chem 72:147–150
Lee WJ, Yoon JR, Park KJ, Chung KM (2000) Fermentation of corn and wheat with supplementation of inactive dry brewer’s yeast. J Am Soc Brew Chem 58:155–159
Levine AS, Fellers CR (1940) Action of acetic acid on food spoilage microorganisms. J Bacteriol 39:499–515
Madshus IH (1988) Regulation of intracellular pH in eukaryotic cells. Biochem J 250:1–8
Maiorella B, Blanch HW, Wilke CR (1983) By-product inhibition effects on ethanolic fermentation by Saccharomyces cerevisiae. Biotechnol Bioeng 25:103–121. doi:10.1002/bit.260250109
Mojovic L, Nikolic S, Rakin M, Vukasinovic M (2006) Production of bioethanol from corn meal hydrolyates. Fuel 85:1750–1755. doi:10.1016/j.fuel.2006.01.018
Naidu K, Singh V, Johnston DB, Rausch KD, Tumbleson ME (2007) Effects of ground corn particle size on ethanol yield and thin stillage soluble solids. Cereal Chem 84:6–9. doi:10.1094/CCHEM-84-1-0006
Narendranath NV, Thomas KC, Ingledew WM (2001) Acetic acid and lactic acid inhibition of growth of Saccharomyces cerevisiae by different mechanisms. J Am Soc Brew Chem 59:187–194
Narendranath NV, Thomas KC, Ingledew WM (2001) Effects of acetic acid and lactic acid on growth of Saccharomyces cerevisiae in a minimal medium. J Ind Microbiol Biotechnol 26:171–177. doi:10.1038/sj.jim.7000090
Pampulha ME, Loureiro V (1989) Interaction of the effects of acetic acid and ethanol on inhibition of fermentation in Saccharomyces cerevisiae. Biotechnol Lett 11:269–274. doi:10.1007/BF01031576
Pampulha ME, Loureiro-Dias MC (1989) Combined effect of acetic acid, pH and ethanol on intracellular pH of fermenting yeast. Appl Microbiol Biotechnol 31:547–550. doi:10.1007/BF00270792
Pampulha ME, Loureiro-Dias MC (1990) Activity of glycolytic enzymes of Saccharomyces cerevisiae in the presence of acetic acid. Appl Microbiol Biotechnol 34:375–380. doi:10.1007/BF00170063
Pampulha ME, Loureiro-Dias MC (2000) Energetics of the effect of acetic acid on growth of Saccharomyces cerevisiae. FEMS Microbiol Lett 184:69–72. doi:10.1111/j.1574-6968.2000.tb08992.x
Phowchinda O, Delia-Dupuy ML, Strehaiano P (1995) Effects of acetic acid on growth and fermentative activity of Saccharomyces cerevisiae. Biotechnol Lett 17:237–242. doi:10.1007/BF00127996
Ramos S, Balbib M, Raposo M, Valle E, Pardo LA (1989) The mechanism of intracellular acidification induced by glucose in Saccharomyces cerevisiae. J Gen Microbiol 64:91–99
Renewable Fuels Association (RFA) (2008) Changing the climate: ethanol industry outlook 2008. http://www.ethanolrfa.org/media/pdf/outlook_2008.pdf. Accessed on 6 March 2008
Russell I (2003) Understanding yeast fundamentals. In: Jacques KA, Lyons TP, Kelsall DR (eds) The alcohol textbook, 4th edn. Nottingham University Press, Nottingham, pp 85–119
Singh V, Batie CJ, Aux GW, Rausch KD, Miller C (2006) Dry-grind processing of corn with endogenous liquefaction enzymes. Cereal Chem 83:317–320. doi:10.1094/CC-83-0317
Singh V, Graeber JV (2005) Effect of corn hybrid variability and planting location on ethanol production. Trans ASAE 48:709–714
Taherzadeh MJ, Niklasson C, Liden G (1997) Acetic acid—friend or foe in anaerobic batch conversion of glucose to ethanol by Saccharomyces cerevisiae? Chem Eng Sci 15:2653–2659. doi:10.1016/S0009-2509(97)00080-8
Taipa MA, Cabral JMS, Santos H (1993) Comparison of glucose fermentation by suspended and gel-entrapped yeast cells: an in vivo nuclear magnetic resonance study. Biotechnol Bioeng 41:647–653. doi:10.1002/bit.260410607
Thomas KC, Dhas A, Rossnagel BG, Ingledew WM (1995) Production of fuel alcohol from hull-less barley by very high gravity technology. Cereal Chem 72:360–364
Thomas KC, Hynes SH, Ingledew WM (2002) Influence of medium buffering capacity on inhibition of Saccharomyces cerevisiae growth by acetic and lactic acids. Appl Environ Microbiol 68:1616–1623. doi:10.1128/AEM.68.4.1616-1623.2002
Thomas KC, Ingledew WM (1990) Fuel alcohol production: effects of free amino nitrogen on fermentation of very-high-gravity wheat mashes. Appl Environ Microbiol 56:2046–2050
United States Department of Agriculture (USDA) (2008) Crop production 2007 summary. http://usda.mannlib.cornell.edu/usda/current/CropProdSu/CropProdSu-01-11-2008.pdf. Accessed on 6 March 2008
Valli M, Sauer M, Branduardi P, Borth N, Porro D, Mattanovich D (2005) Intracellular pH distribution in Saccharomyces cerevisiae cell populations, analyzed by flow cytometry. Appl Environ Microbiol 71:1515–1521. doi:10.1128/AEM.71.3.1515-1521.2005
Wang S, Ingledew WM, Thomas KC, Sousulski K, Sosulski FW (1999) Optimization of fermentation temperature and mash specific gravity for fuel alcohol production. Cereal Chem 76:82–86. doi:10.1094/CCHEM.1999.76.1.82
Wang S, Thomas KC, Ingledew WM, Sosulski K, Sosulski FW (1997) Rye and triticale as feedstock for fuel ethanol production. Cereal Chem 74:621–625. doi:10.1094/CCHEM.1997.74.5.621
Wu X, Wang D, Bean SR, Wilson JP (2006) Ethanol production from pearl millet using Saccharomyces cerevisiae. Cereal Chem 83:127–131. doi:10.1094/CC-83-0127
Wu X, Zhao R, Bean SR, Seib PA, McLaren JS, Madl RL et al (2007) Factors impacting ethanol production from grain sorghum in the dry-grind process. Cereal Chem 84:130–136. doi:10.1094/CCHEM-84-2-0130
Wu X, Zhao R, Wang D, Bean SR, Seib PA, Tuinstra MR et al (2006) Effects of amylose, corn protein and corn fiber contents on production of ethanol from starch rich media. Cereal Chem 83:569–575. doi:10.1094/CC-83-0569
Zhan X, Wang D, Bean SR, Mo X, Sun XS, Boyle D (2006) Ethanol production from supercritical-fluid-extrusion cooked sorghum. Ind Crops Prod 23:304–310. doi:10.1016/j.indcrop.2005.09.001
Zhao R, Bean S, Wang D, Park SH, Schober TJ, Wilson J (2008) Small-scale mashing procedure for predicting ethanol yield of sorghum grain. J Cereal Sci 48 (in press)
Acknowledgments
This project was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number 2004-35504-14808. Authors would like to thank Novozymes Inc. for providing Liquozyme SC DS and Spirizyme Fuel, and Fermentis of S.I. Lesaffre for the active dry yeast used in this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Names are necessary to report factually on available data; however, the U.S. Department of Agriculture neither guarantees nor warrants the standard of the product, and use of the name by the U.S. Department of Agriculture implies no approval of the product to the exclusion of others that may also be suitable.
Rights and permissions
About this article
Cite this article
Zhao, R., Bean, S.R., Crozier-Dodson, B.A. et al. Application of acetate buffer in pH adjustment of sorghum mash and its influence on fuel ethanol fermentation. J Ind Microbiol Biotechnol 36, 75–85 (2009). https://doi.org/10.1007/s10295-008-0474-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0474-y