Abstract
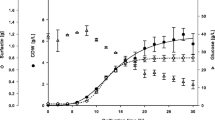
The main characteristic of biosurfactants is their property of reducing the superficial and interfacial tension between two immiscible liquids of different polarities. The main obstacle to the application of biosurfactants is the high production costs, the use of alternative substrates being indicated to solve this problem. This work report the production of biosurfactant by Bacillus subtilis LB5a on a pilot scale using cassava wastewater as the substrate, and the study of the parameters related to its production. The cassava wastewater was heated, centrifuged and poured into a 40-liter batch pilot bioreactor adapted for simultaneous foam collection during the fermentative process. The temperature was maintained at 35 °C, agitation at 150 rpm and aeration 0.38 vvm during the first 12 h, and 0.63 vvm for the rest of the process. Samples of liquid fermentate were collected at regular intervals for the analysis of total carbohydrates, reducing sugars, pH, CFU/mL count and superficial tension. The foam was centrifuged and the biosurfactant purified. The kinetic data of the process showed that both the microbial population, which reached a maximum after about 24 h, and the foam production of 10.6 L, peaked between 24 and 36 h, coinciding with the greatest production of biosurfactant. The yield of semi-purified surfactant in the foam was 2.4 g/L. The superficial tension of the medium was reduced from 51 to 27 mN/m and the critical micellar concentration was 11 mg/L, which, in principle, characterizes it as a good tensoactive agent. As a function of its composition and productivity, cassava wastewater was identified as a good substrate for the production of the biosurfactant.




Similar content being viewed by others
References
Ahimou F, Jaccques P, Deleu M (2000) Surfactin and iturin A effects on Bacillus subtilis surface hydrophobicity. Enzyme Microb Technol 27:749–754. doi:10.1016/S0141-0229(00)00295-7
AOAC (1995) Official method of analysis, 16th edn. AOAC International, Arlington
Banat IM, Makkar RS, Cameotra SS (2000) Potential commercial applications of microbial surfactants. Appl Microbiol Biotechnol 53:495–508. doi:10.1007/s002530051648
Barros FFC, Quadros CP, Maróstica MR Jr, Pastore GM (2007) Surfactina: propriedades químicas, tecnológicas e funcionais para aplicação em alimentos. Quim Nova 30:409–414
Bognolo G (1999) Biossurfactants as emulsifying agents for hydrocarbons. Colloids Surf 12:41–52
Bremmer JM, Keeney DR (1965) Steam-distillation methods for determination of ammonium, nitrate and nitrite. Anal Chim Acta 32:485–495. doi:10.1016/S0003-2670(00)88973-4
Cameotra SS, Makkar RS (1998) Synthesis of biosurfactants in extreme conditions. Appl Microbiol Biotechnol 50:520–529. doi:10.1007/s002530051329
Cereda MP (2005) Produtos e subprodutos. In: Souza LS, Farias ARN, Mattos PLP, Fukuda WMG (eds) Processamento e utilização da mandioca. Embrapa Mandioca e Fruticultura Tropical, Cruza das Almas
Cooper DG, Macdonald CR, Duff SJB, Kosaric N (1981) Enhanced production of surfactin from Bacillus subtilis by continuous product removal and metal cation additions. Appl Environ Microbiol 42:408–412
Daniels L, Hanson R, Phyllips JA (1994) Chemical analysis. In: Gerhardt P, Murray RGE, Wood WA, Krieg NR (eds) Methods for general and molecular bacteriology. American Society for Microbiology, Washington
Davis DA, Lynch HC, Varley J (2001) The application of foaming for the recovery of surfactin from Bacillus subtilis ATCC 21332 cultures. Enzyme Microb Technol 28:346–354. doi:10.1016/S0141-0229(00)00327-6
Davis DA, Lynch HC, Varley J (1999) The production of surfactin in batch culture by Bacillus subtilis ATCC 21332 is strongly influenced by the conditions of nitrogen metabolism. Enzyme Microb Technol 25:322–329. doi:10.1016/S0141-0229(99)00048-4
Deleu M, Paquot M, Nylander T (2005) Fengycin interaction with lipid monolayers at the air-aqueous interface-implications fopr the effect of fengycin on biological membranes. J Colloid Interface Sci 283:358–365. doi:10.1016/j.jcis.2004.09.036
Deleu M, Razafindralambo H, Popineau Y, Jacques P, Thonart P, Paquot M (1999) Interfacial and emulsifying properties of lipopeptides from Bacillus subtilis. Colloids Surf A Physicochem Eng Asp 152:3–10. doi:10.1016/S0927-7757(98)00627-X
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potencial. Microbiol Mol Biol Rev 61:47–64
Desai JD, Desai AJ (1993) Production of biosurfactants. In: Kosaric N (ed) Biosurfactants: production, properties, applications. Marcel Decker, New York
EPA—US Environmental Protection Agency (1994) SW-846 Tests methods for evaluating solid wastes: Physical and chemicam methods. Washington
Fox SL, Bala GA (2000) Production of surfactant from Bacillus subtilis ATCC 21332 using potato substrates. Bioresour Technol 75:235–240. doi:10.1016/S0960-8524(00)00059-6
Gu X, Zheng Z, Yu H, Wang J, Liang F, Liu R (2005) Optimization of medium constituents for a novel lipopeptide production by Bacillus subtilis MO-01 by a response surface method. Process Biochem 40:3196–3201. doi:10.1016/j.procbio.2005.02.011
Healy MG, Devine CM, Murphy R (1996) Microbial production of biosurfactants. Resour Conserv Recycling 18:41–57. doi:10.1016/S0921-3449(96)01167-6
Huang H, Ridgway D, Gu T, Moo-Young M (2004) Enhanced amylase production by Bacillus subtilis using a dual exponential feeding strategy. Bioprocess Biosyst Eng 27:63–69. doi:10.1007/s00449-004-0391-z
Kim H, Yoon B, Lee C, Suh H, Oh H, Katsuragi T et al (1997) Production and properties of a lipopeptide biosurfactant from Bacillus subtilis C9. J Ferment Bioeng 84:41–46. doi:10.1016/S0922-338X(97)82784-5
Kluge B, Vater J, Salnikow J, Eckart K (1988) Studies on the biosynthesis of surfactin, a lipopeptide antibiotic from Bacillus subtilis ATCC 21332. FEBS Lett 231:107–110. doi:10.1016/0014-5793(88)80712-9
Kowall M, Vater J, Kluge B, Stein T, Franke P, Ziessow D (1998) Separation and characterization of surfactin isoforms produced by Bacillus subtilis OKB 105. J Colloid Interface Sci 204:1–8. doi:10.1006/jcis.1998.5558
Lang S (2002) Biological amphiphiles (microbial surfactantes). Curr Opin Colloid Interface Sci 7:12–20. doi:10.1016/S1359-0294(02)00007-9
Lin SC (1996) Biosurfactants: recents advances. J Chem Technol Biotechnol 66:109–120. doi:10.1002/(SICI)1097-4660(199606)66:2<109::AID-JCTB477>3.0.CO;2-2
Lin SC, Carswell KS, Sharma MM, Georgiou G (1994) Continuous production of the lipopeptide biosurfactant of Bacillus licheniformis JF-2. Appl Microbiol Biotechnol 41:281–285. doi:10.1007/BF00221219
Maier RM (2003) Biosurfactants: evolution and diversity in bacteria. Adv Appl Microbiol 52:101–121. doi:10.1016/S0065-2164(03)01004-9
Makkar RS, Cameotra SS (2002) An update on the use of unconventional substrates for biosurfactant production and their new applications. Appl Microbiol Biotechnol 58:428–434. doi:10.1007/s00253-001-0924-1
Makkar RS, Cameotra SS (1997) Biosurfactant production by a thermophilic Bacillus subtilis strain. J Ind Microbiol Biotechnol 18:37–42. doi:10.1038/sj.jim.2900349
Makkar RS, Cameotra SS (1999) Structural characterization of a biosurfactant poduced by Bacillus subtilis at 45 °C. J Surfactants Deterg 2:367–372
Mulligan CN, Gibbs BF (1990) Recovery of biosurfactants by ultrafiltration. J Tech Biotechnol 47:23–29
Mulligan C (2005) Environmental applications for biosurfactants. Environ Pollut 133:183–198. doi:10.1016/j.envpol.2004.06.009
Nitschke M, Ferraz C, Pastore GM (2004) Selection of microorganisms for biosurfactant production using agro industrial wastes. Braz J Microbiol 35:81–85. doi:10.1590/S1517-83822004000100013
Nitschke M, Haddad R, Costa GAN, Gilioli R, Meurer EC, Gatti MS et al (2004) Strutural characterization and biological properties of a lipopeptide surfactant produced by Bacillus subtilis on cassava wastewater medium. Food Sci Biotechnol 13:591–596
Nitschke M, Pastore GM (2006) Production and properties of a surfactant obtained from Bacillus subtilis grown on cassava wastewater. Bioresour Technol 97:336–341. doi:10.1016/j.biortech.2005.02.044
Pantaroto S, Cereda MP (2000) Linamarina e sua decomposição no ambiente. In: Cereda MP (ed) Manejo, uso e tratamento de subprodutos da industrialização da mandioca, vol 4. Fund, Cargill
Pastore GM, Santos CFC, Nitschcke M (2003) Processo de Produção de Biosuractante por Bacillus subtilis, utilizando resíduo da indústria de mandioca. Br Pat PI 0303853-0.
Peypoux F, Bonmatin JM, Labbé H, Das BC, Ptak M, Michel G (1991) Isolation and characterization of a new variant of surfactin, the [Val7] surfactin. Eur J Biochem 202:101–106. doi:10.1111/j.1432-1033.1991.tb16349.x
Peypoux F, Bonmatin JM, Wallach J (1999) Recents trends in biochemistry of surfactin. Appl Microbiol Biotechnol 51:553–563. doi:10.1007/s002530051432
Razafindralambo H, Paquot M, Baniel A, Popineau Y, Hbid C, Jacques P et al (1996) Foaming properties of surfactin, a lipopeptide biosurfactant from Bacillus subtilis. J Am Oil Chem Soc 73:149–151. doi:10.1007/BF02523463
Reiling HE, Thanei-Wyss U, Guerra-Santos LH, Hirt R, Käppeli O, Fiechter A (1986) Pilot plant production of rhaminolipid biosurfactant by Pseudomonas aeruginosa. Appl Environ Microbiol 51:985–989
Sandrin C, Peipoux F, Michel G (1990) Coproduction of surfactin and iturin A lipopeptides with surfactant and antifungal properties by Bacillus subtilis. Biotechnol Appl Biochem 12:370–375
Sheppard JD, Cooper DG (1991) The response of Bacillus subtilis ATCC 21332 to manganese during continuous-phased growth. Appl Microbiol Biotechnol 35:72–76. doi:10.1007/BF00180639
Sheppard JD, Mulligan CN (1987) The production of surfactin by Bacillus subtilis grown on peat hydrolysate. Appl Microbiol Biotechnol 27:110–116. doi:10.1007/BF00251931
Somogyi M (1945) A new reagent for determination of sugars. J Biol Chem 160:61–68
Thoma J, Brother C, Spradlin J (1970) Subsite mapping of enzymes. Studies on Bacillus subtilis amylase. Biochemistry 9:1768–1775. doi:10.1021/bi00810a016
Thompson DN, Fox SL, Bala GA (2000) Biosurfactants from potato process effluents. Appl Biochem Biotechnol 84:917–929. doi:10.1385/ABAB:84-86:1-9:917
Vater J (1986) Lipopeptides, an attractive class of microbial surfactants. Prog Colloid Polym Sci 72:12–18. doi:10.1007/BFb0114473
Veenanadig NK, Gowthaman MK, Karath NGK (2000) Scale up for the production of biosurfactant packed column bioreactor. Bioprocess Eng 32:95–99. doi:10.1007/s004490050017
Wei Y, Chu I (2002) Mn2+ improves surfactin production by Bacillus subtilis. Biotechnol Lett 24:479–482. doi:10.1023/A:1014534021276
Wei Y, Chu I (2004) Optimizing iron supplement strategies for enhanced surfactin production with Bacillus subtilis. Biotechnol Prog 20:979–983. doi:10.1021/bp030051a
Yeh M, Wei Y, Chang J (2006) Bioreactorndesign for enhanced carrier-assisted surfactin production with Bacillus subtilis. Process Biochem 41:1799–1805. doi:10.1016/j.procbio.2006.03.027
Acknowledgments
Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Counsil of Technological and Scientific Development)—CNPq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barros, F.F.C., Ponezi, A.N. & Pastore, G.M. Production of biosurfactant by Bacillus subtilis LB5a on a pilot scale using cassava wastewater as substrate. J Ind Microbiol Biotechnol 35, 1071–1078 (2008). https://doi.org/10.1007/s10295-008-0385-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0385-y