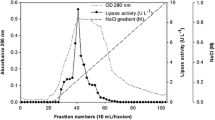
Abstract
An isolate exhibiting high extracellular lipolytic activity was identified as Bacillus subtilis by 16S rRNA gene sequence analysis. The enzyme activity of the isolate was improved by using different concentrations of lipidic carbon sources such as vegetable oils, fatty acids and triglycerides. Lipolytic activity was assayed spectrophotometrically using p-nitrophenyl palmitate. One percent (v/v) of sesame oil provided the highest activity with 80 and 98% enhancements with respect to 1% (v/v) concentrations of linoleic acid and triolein as the favored fatty acid and triglyceride, respectively. Glucose presented a repressive effect on lipase production. Lipase secreted by B. subtilis was partially purified by ultrafiltration and anion exchange chromatography; and the purified enzyme was tested for its residual activity in the presence of EDTA, SDS, Triton X-100, Tween 20, Tween 80 and protease. The present work reports, for the first time, that the lipolytic activity of a B. subtilis strain can be improved by using inexpensive vegetable oils; and also that B. subtilis lipase is suitable for use in detergents.






Similar content being viewed by others
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cernia E, Deflini M, Cocco ED, Palloci C, Soro S (2002) Investigation of lipase-catalysed hydrolysis of naproxen methyl ester: use NMR spectrescopy methods to study substrate-enzyme interaction. Bioorg Chem 30:276–284
Cizmeci M, Musavi A, Kayahan M, Tekin A (2005) Monitoring of hydrogenation with various catalyst ratios. J Am Oil Chem Soc 82:925–929
Doetsch RN (1981) Determinative methods of light microscopy. In: Gerhardt P (ed) Manuel for general bacteriology. American Society for Microbiology, Washington DC, pp 21–23
Drancourt M, Raoult D (2005) Sequence-based identification of new bacteria: a proposition for creation on an orphan bacterium repository. J Clin Microbiol 43(9):4311–4315
Eggert T, Pencreac`h G, Douchet I, Verger R, Jaeger KE (2000) A novel extracellular esterase from Bacillus subtilis and its conversion to a monoacylglycerol hydrolase. Eur J Biochem 267:6459–6469
Eggert T, Pouderoyenb G, Dijkstrab BW, Jaegera KE (2001) Lipolytic enzymes LipA and LipB from Bacillus subtilis differ regulation of gene expression, biochemical properties, and three-dimensional structure. FEBS Lett 502:89–92
Eggert T, Pouderoyen G, Pencreac’h G, Douchet U, Verger R, Dijkstra BW, Jaeger KE (2002) Biochemical properties and three-dimensional structures of two extracellular lipolytic enzymes from Bacillus subtilis. Colloids Surf B Biointerfaces 26:37–46
Gupta R, Gupta N, Rathi P (2004) Bacterial lipases: an overview of production, purification and biochemical properties. Appl Microbiol Biotechnol 64:763–781
Hasan F, Shah AA, Hameed A (2006) Industrial applications of microbial lipases. Enzyme Microb Technol 39:235–251
Hung T, Gridhar R, Chiou S, Wu W (2003) Binary immobilization of Candida rugosa lipase on chitosan. J Mol Catal B Enzym 26:69–78
Krieg NR (1981) Enrichment and isolation. In: Gerhardt P (ed) Manuel for General Bacteriology. American Society for Microbiology, Washington DC, pp 112–142
Kumar S, Kikon K, Upadhyay A, Kanwar SS, Gupta R (2005) Production, purification, and characterization of lipase from thermophilic and alkaliphilic Bacillus coagulans BTS-3. Protein Expr Purif 41(1):38–44
Meghji K, Ward OP, Araujo A (1990) Production, purification and properties of extracellular carboxyl esterases from Bacillus subtilis NRRL 365. Appl Environ Microbiol 56(12):3735–3740
Prasanth Kumar MP, Valsa AK (2007) Optimization of culture media and cultural conditions for the production of extracellular lipase by Bacillus coagulans. Indian J Biotechnol 6(1):114–117
Sanchez M, Prim N, Randez-Gil F, Pastor FIJ, Diaz P (2002) Engineering of baker’s yeasts, E. coli and Bacillus hosts for the production of Bacillus subtilis Lipase A. Biotechnol Bioeng 78(3):339–345
Sekhon A, Dahiya N, Tewari RP, Hoondal GS (2006) Engineering of baker’s yeasts, Ecoli and Bacillus hosts for the production of Bacillus subtilis Lipase A. Indian J Biotechnol 5(2):179–183
Sharma R, Chistib Y, Banerjee UC (2001) Production, purification, characterization and applications of lipases. Biotechnol Adv 19:627–662
Standard methods for analysis of oils, fats, and derivatives (1987) 7th ed. Blackwell Scientific, Oxford, IUPAC Method 2.301
Takaç S, Elmas S, Çalık P, Özdamar HT (2000) Separation of the protease enzymes of Bacillus licheniformis from fermentation medium by crossflow ultrafiltration. J Chem Technol Biotechnol 75(6):491–499
Acknowledgments
The authors gratefully acknowledge the financial supports provided by Ankara University Biotechnology Institute (Project No: 2005-164 of the SPO Project No: 2001K120240) and by Ankara University Scientific Research Projects Unit (Project No: 2007-0745004-HPD). The suggestions and experimental support from Prof. Dr. G. Dönmez and Sevgi Ertuğrul (Ankara University Department of Biology) on the isolation of microorganism are gratefully recognized. Sincere thanks are extended to Assoc. Prof. Dr. H. Özdağ and G. Özsöz (Ankara University Biotechnology Institute) for their technical and experimental assistances on 16S rRNA gene sequencing, respectively. Analyses for fatty acid compositions of vegetable oils were kindly performed by Prof. Dr. A. Tekin (Ankara University, Department of Food Engineering).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takaç, S., Marul, B. Effects of lipidic carbon sources on the extracellular lipolytic activity of a newly isolated strain of Bacillus subtilis . J Ind Microbiol Biotechnol 35, 1019–1025 (2008). https://doi.org/10.1007/s10295-008-0377-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0377-y