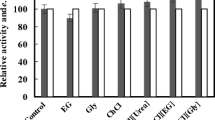
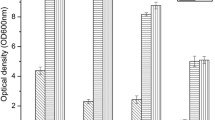
Abstract
Ethyl(R)-4-chloro-3-hydroxybutanoate ((R)-CHBE) are obtained by cetyltrimetylammonium bromide (CTAB) permeabilized fresh brewer’s yeast whole cells bioconversion of ethyl 4-chloro-3-oxobutanoate (COBE ) in the presence of allyl bromide. The results showed that the activities of alcohol dehydrogenase (ADH) and glucose-6-phosphate dehydrogenase (G6PDH) in CTAB permeabilized brewer’s yeast cells increased 525 and 7.9-fold, respectively, compared with that in the nonpermeabilized cells and had high enantioselectivity to convert COBE to (R)-CHBE. As one of co-substrates, glucose-6-phosphate was preprepared using glucose phosphorylation by hexokinase-catalyzed of CTAB permeabilized brewer’s yeast cells. In a two phase reaction system with n-butyl acetate as organic solvent and with 2-propanol and glucose-6-phosphate as co-substrates, the highest (R)-CHBE concentration of 447 mM was obtained with 110–130 g/l of the CTAB permeabilized cells at optimized pH, temperature, feeding rate and the shake speed of 125 r/min. The yield and enantiomeric excess (ee) of (R)-CHBE reached 99.5 and 99%, respectively, within 6 h.




Similar content being viewed by others
References
Shimizu S, Kataoka M, Kita K (1998) Chiral alcohol synthesis with microbial carbonyl reductases in a water-organic solvent two-phase system. Ann NYAcad Sci 864:87–95
Wang G, Hollingsworth RI (1999) Synthetic routes to l-carnitine and l-gamma-amino-beta-hydroxybutyric acid from (S)-3-hydroxybutyrolactone by functional group priority switching. Tetrahedron. Asymmetry 10:1895–1901
Forni A, Caselli E, Prati F, Bucciarelli M, Torre G (2002) Highly enantioselective reduction of ethyl 4-chloro-3-oxobutanoate to l- and D-3-hydroxyesters with baker’s yeast. Arkivoc (xi):45–53
Shimizu S, Kataoka M, Katoh M, Morikawa T, Miyoshi T, Yamada H(1990) Stereoselective Reduction of ethyl 4-chloro-3-oxobutanoate by a microbial aldehyde reductase in an organic solvent-water diphasic system. Appl Environ Microbiol 56(8):2374–2377
Kataoka M, Yamamoto K, Kawabata H, Wada M, Kita K,Yanase H,Shimizu S (1999) Stereoselective reduction of ethyl 4-chloro-3-oxobutanoate by Escherichia coli transformant cells coexpressing the aldehyde reductase and glucose dehydrogenase genes. Appl Microbiol Biotechnol 51(4):486–490
Liu Y, Xu ZN, Jing KJ, Jiang XX, Lin JP, Wang F, Cen PL (2005) Asymmetric reduction of ethyl 4-chloro-3-oxobutanoate to ethyl (R)-4-chloro-3-hydroxybutanoate with two co-existing, recombinant Escherichia coli strains. Biotechnol Lett 27(2):119–125
Bertau M, Burli M (2000) Enantioselective microbial reduction with baker’s yeast on an industrial scale. Chimia 59:503–507
Chin-Joe I, Straathof AJJ, Pronk JK, Jongejan JA, Heijnen JJ (2001) Influence of the ethanol and glucose supply rate on the rate and enantioselectivity of 3-oxo ester reduction by baker’s yeast. Biotechnol Bioeng 75:29–38
Novak J, Zarevucka M, Wimmer Z, Tykva R (2001) A comparison of the enantioselective reduction potential of strains of Saccharomyces cerevisiae towards a selected ketone. Biotechnol Lett 23:1517–1522
Gowda LR, Joshi MS, Bhat SG (1988) In situ assay of intracellular enzyme of yeast (Kluyveromyces tragilis) by digitonin permeabilization of cell membrane. Anal Biochem 175:531–536
Gowda LR, Bachhawat N, Bhat SG (1991) Permeabilization of bakers’ yeast by cetyltrimethylammonium bromide for intracellular enzyme catalysis. Enzyme Microb Technol 13:154–157
Smart KA, Chambers KM, Lambert I, Jenkins C (1999) Use of methylene violet staining procedures to determine yeast viability and vitality. J Am Soc Brew Chem 57(1):18–23
Leisola M, Linko M (1991) Preparation and purification of fructose-1,6-diphosphate. Acta Chem Scand B28 (5):555–558
Colowick SP, Kaplan NO (1982) Glucose-6-phosphate: hexokinase-catalyzed phosphorylation of glucose. In: Wood WA (ed) Methods in enzymology. vol. 89, carbohydrate metabolism (Part D). Academic, New York, pp 112–113
Engelking H, Pfaller R, Wich G, Weuster-Botza D (2004) Stereoselective reduction of ethyl 4-chloro acetoacetate with recombinant Pichia pastoris. Tetrahedron Asymmetry 15:3591–3593
Acknowledgments
This work was supported by the special fund of three items of expenditure on science and technology department of Central District in Chongqing. We also thank the Chongqing University of Medical Sciences for partial financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, MA., Wei, YM., Zhao, L. et al. Bioconversion of ethyl 4-chloro-3-oxobutanoate by permeabilized fresh brewer’s yeast cells in the presence of allyl bromide. J Ind Microbiol Biotechnol 34, 151–156 (2007). https://doi.org/10.1007/s10295-006-0179-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-006-0179-z