Abstract
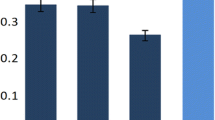
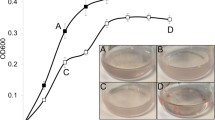
Pseudomonas species were used in bioremediation technologies. In situ conditions, such as marine salinity, could limit the degradation of hydrocarbons and aromatic compounds by the bacteria. Biofilm ability to tolerate environmental stress could be used to increase biorestoration. In this report, we used scanning confocal laser microscopy and microtiter dish assay to analyse the impact of hyperosmotic stress on biofilm formation by Pseudomonas aeruginosa. We used benzoate as the sole carbon source and the effect of the stress on its degradation was also studied. Hyperosmotic shock inhibited the biofilm development and decreased the degradation of benzoate. The osmoprotectant glycine betaine partially restored both the biofilm formation and benzoate degradation, suggesting that it could be used as a complement in bioremediation processes.



Similar content being viewed by others
References
Bloemberg GV, O’Toole GA, Lugtenberg BJ, Kolter R (1997) Green fluorescent protein as a marker for Pseudomonas spp. Appl Environ Microbiol 63:4543–4551
Bremer E, Krämer R (2000) Coping with osmotic challenges: osmoregulation through accumulation and release of compatible solutes in bacteria. Bacterial stress responses, chapter 6, pp 79–97
Csonka LN (1989) Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev 53:121–147
Davey ME, Caiazza NC, O’Toole GA (2003) Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J Bacteriol 185:1027–1036
De Kievit TR, Gillis R, Marx S, Brown C, Iglewski H (2001) Quorum-sensing genes in Pseudomonas aeruginosa biofilms: their role and expression patterns. Appl Environ Microbiol 67:1865–1873
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61:47–64
Harayama S, Kok M, Neidle EL (1992) Functional and evolutionary relationships among diverse oxygenases. Annu Rev Microbiol 46:565–601
Holloway BW, Krishnapillai V, Morgan AF (1979) Chromosomal genetics of Pseudomonas. Microbiol Rev 43:73–102
Lang S, Wullbrandt D (1999) Rhamnose lipids—biosynthesis, microbial production and application potential. Appl Microbiol Biotechnol 51:22–32
Mah TC, O’Toole GA (2001) Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9:34–39
Manetas Y, Petropoulou Y, Karabourniotis G (1986) Compatible solutes and their effects on phosphoenolpyruvate carboxylase of C4-halophytes. Plant Cell Environ 9:145–151
Riis V, Kleinsteuber S, Babel W (2003) Influence of high salinities on the degradation of diesel fuel by bacterial consortia. Can J Microbiol 49:713–721
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schwab KB, Gaff DF (1986) Sugar and ion content in leaf of several drought tolerant plants under water stress. J Plant Physiol 125:257–265
Stanley NR, Lazazzera BA (2004) Environmental signals and regulatory pathways that influence biofilm formation. Mol Microbiol 52:917–924
Talibart R, Jebbar M, Gouesbet G, Himdi-Kabbab S, Wroblewski H, Blanco C, Bernard T (1994) Osmoadaptation in rhizobia: ectoine-induced salt tolerance. J Bacteriol 176:5210–5217
Watnick P, Kolter R (2000) Biofilm, city of microbes. J Bacteriol 182:2675–2679
Acknowledgements
This work was supported by the Region Bretagne, FEDER funds, and the Ministère de la Recherche et de la Technologie (RITMER grant and doctoral fellowships to A.B. and F. D.). We thank G. A. O’Toole for the kind gift of plasmid pSMC21. We thank A. Dufour for his critically reading manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Alexis Bazire and Farès Diab contributed equally to this work.
Rights and permissions
About this article
Cite this article
Bazire, A., Diab, F., Jebbar, M. et al. Influence of high salinity on biofilm formation and benzoate assimilation by Pseudomonas aeruginosa . J Ind Microbiol Biotechnol 34, 5–8 (2007). https://doi.org/10.1007/s10295-006-0087-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-006-0087-2