Abstract
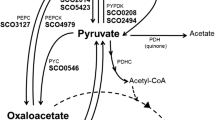
In most bacteria acetate assimilation is accomplished via the glyoxylate pathway. Isocitrate lyase (ICL) and malate synthase (MS) are two key enzymes of this pathway, which results in the net generation of one molecule of succinyl-CoA from two acetyl-CoA molecules. Genetic and biochemical data have shown that genes encoding these key enzymes are present in streptomycetes, yet there has been no clear demonstration of the importance of these genes to acetate assimilation. In fact, for Streptomyces collinus an alternative butyryl-CoA pathway has been shown to be critical for growth on acetate as a sole carbon source. Crotonyl-CoA reductase (CCR) is a key enzyme in this pathway and catalyzes the last step of the conversion of 2-acetyl-CoA molecules to butyryl-CoA. In Streptomyces cinnamonensis C730.1, it has been shown that CCR and this butyryl-CoA pathway provide the majority of methylmalonyl-CoA and ethylmalonyl-CoA for monensin A biosynthesis in an oil-based fermentation medium. We have cloned a MS homologue gene from this strain. Reverse transcription and direct enzyme assays demonstrated that neither this nor other MS genes were expressed during fermentation in an oil-based fermentation of either the C730.1 or L1 strain (a ccr mutant). Similarly, no ICL activity could be detected. The C730.1 but not the L1 strain was able to grow on acetate as a sole carbon source. The Streptomyces coelicolor aceA and aceB2 genes encoding ICL and MS were cloned into a Streptomyces expression plasmid (a derivative of pSET152) to create pExIM1. Enzyme assays and transcript analyses demonstrated expression of both of these proteins in C730.1/pExIM1 and L1/pExIM1 grown in an oil-based fermentation and tryptic soy broth media. Nonetheless, L1/pExIM1, like L1, was unable to grow on acetate as a sole carbon source, and was unable to efficiently generate precursors for monensin A biosynthesis in an oil-based fermentation, indicating that the additional presence of these two enzyme activities does not permit a functional glyoxylate cycle to occur. UV mutagenesis of S. cinnamonensis L1 and L1/pExIM1 led to mutants which were able to grow efficiently on acetate despite a block in the butyryl-CoA pathway. Analysis of enzyme activity and monensin production from these mutants in an oil-based fermentation demonstrated that neither the glyoxylate cycle nor the butyryl-CoA pathway function, suggesting the possibility of alternative pathways of acetate assimilation.








Similar content being viewed by others
References
Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, Bateman A, Brown S, Chandra G, Chen CW, Collins M, Cronin A, Fraser A, Goble A, Hidalgo J, Hornsby T, Howarth S, Huang CH, Kieser T, Larke L, Murphy L, Oliver K, O’Neil S, Rabbinowitsch E, Rajandream MA, Rutherford K, Rutter S, Seeger K, Saunders D, Sharp S, Squares R, Squares S, Taylor K, Warren T, Wietzorrek A, Woodward J, Barrell BG, Parkhill J, Hopwood DA (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147
Chan M, Sim TS (1998) Malate synthase from Streptomyces clavuligerus NRRL3585: cloning, molecular characterization and its control by acetate. Microbiology 144(Pt 11):3229–3237
Cozzone AJ (1998) Regulation of acetate metabolism by protein phosphorylation in enteric bacteria. Annu Rev Microbiol 52:127–164
Han L, Reynolds KA (1997) A novel alternate anaplerotic pathway to the glyoxylate cycle in streptomycetes. J Bacteriol 179:5157–5164
Hopwood DA, Bibb MJ, Chater KF, Kieser T, Bruton CJ, Kieser HM, Lydiate DJ, Smith CP, Smith JM, Ward JM, Schrempf HS (1985) Genetic manipulation of streptomycetes, a laboratory manual. John Innes Institute, Norwich
Huttner S, Mecke D, Frohlich KU (1997) Gene cloning and sequencing, and enzyme purification of the malate synthase of Streptomyces arenae. Gene 188:239–246
Ikeda H, Ishikawa J, Hanamoto A, Shinose M, Kikuchi H, Shiba T, Sakaki Y, Hattori M, Omura S (2003) Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat Biotechnol 21:526–531
Ivanovsky RN, Krasilnikova EN, Berg IA (1997) A proposed citramalate cycle for acetate assimilation in the purple non-sulfur bacterium Rhodospirillum rubrum. FEMS Microbiol Lett 153:399–404
Kornberg HL (1966) The role and control of the glyoxylate cycle in Escherichia coli. Biochem J 99:1–11
Korotkova N, Chistoserdova L, Kuksa V, Lidstrom ME (2002) Glyoxylate regeneration pathway in the methylotroph Methylobacterium extorquens AM1. J Bacteriol 184:1750–1758
Li C, Florova G, Konstatin A, Reynolds KA (2004) Crotonyl-coenzyme A reductase provides methylmalonyl-CoA precursors for monensin biosynthesis by Streptomyces cinnamonensis in an oil-based extended fermentation. Microbiology 150:3463–3472
Liu H, Reynolds KA (1999) Role of crotonyl coenzyme A reductase in determining the ratio of polyketides monensin A and monensin B produced by Streptomyces cinnamonensis. J Bacteriol 181:6806–6813
Loke P, Sim TS (2000) Molecular cloning, heterologous expression, and functional characterisation of a malate synthase gene from Streptomyces coelicolor A3(2). Can J Microbiol 46:764–769
Loke P, Wee J, Seah K, Sim TS (2001) PCR-mediated screening and cloning of a malate synthase gene from Streptomyces griseus NCIMB 9001. World J Microbiol Biotechnol 17:645–649
Loke P, Goh LL, Seng Soh B, Yeow P, Sim TS (2002) Purification and characterization of recombinant malate synthase enzymes from Streptomyces coelicolor A3(2) and S. clavuligerus NRRL3585. J Ind Microbiol Biotechnol 28:239–243
Omura S, Ikeda H, Ishikawa J, Hanamoto A, Takahashi C, Shinose M, Takahashi Y, Horikawa H, Nakazawa H, Osonoe T, Kikuchi H, Shiba T, Sakaki Y, Hattori M (2001) Genome sequence of an industrial microorganism Streptomyces avermitilis: deducing the ability of producing secondary metabolites. Proc Natl Acad Sci U S A 98:12215–12220
Park SJ, Cotter PA, Gunsalus RP (1995) Regulation of malate dehydrogenase (mdh) gene expression in Escherichia coli in response to oxygen, carbon, and heme availability. J Bacteriol 177:6652–6656
Reynolds KA, O’Hagan D, Gani D, Robinson JA (1988) Butyrate metabolism in streptomycetes. Characterization of a vicinal interchange rearrangement linking isobutyrate and butyrate in Streptomyces cinnamonensis. J Chem Soc Perkin Trans I 3195–3207
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Soh BS, Loke P, Sim TS (2001) Cloning, heterologous expression and purification of an isocitrate lyase from Streptomyces clavuligerus NRRL 3585. Biochim Biophys Acta 1522:112–117
Wallace KK, Bao Z, Dai H, Digate R, Schuler G, Speedie MK, Reynolds KA (1995) Purification of crotonyl CoA reductase from Streptomyces collinus and cloning, sequencing and expression of the corresponding gene in Escherichia coli. Eur J Biochem 233:954–962
Zhang W, Reynolds KA (2001) MeaA, a putative coenzyme B(12)-dependent mutase, provides methylmalonyl coenzyme A for monensin biosynthesis in Streptomyces cinnamonensis. J Bacteriol 183:2071–2080
Acknowledgments
We are grateful to Dr. H. Kieser for providing BAC 19F3 containing the S. coelicolor aceA and aceB. This work was supported by grants from the National Institute of Health (GM 50542) and Eli Lilly. We are grateful to Richard DeMaio and Vic Vinci at Eli Lilly for providing S. cinnamonensis C730.1 and for conditions and media for oil-based extended fermentations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akopiants, K., Florova, G., Li, C. et al. Multiple pathways for acetate assimilation in Streptomyces cinnamonensis . J IND MICROBIOL BIOTECHNOL 33, 141–150 (2006). https://doi.org/10.1007/s10295-005-0029-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-005-0029-4