Abstract
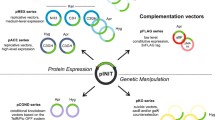
The genus Amycolatopsis is of industrial importance, as its species are known to produce commercial antibiotics. It belongs to the family Pseudonocardiaceae and has an eventful taxonomic history. Initially strains were identified as Streptomyces, then later as Nocardia. However, based on biochemical, morphological and molecular features, the genus Amycolatopsis, containing seventeen species, was created. The development of molecular genetic techniques for this group has been slow. The scarcity of molecular genetic tools including stable plasmids, antibiotic resistance markers, transposons, reporter genes, cloning vectors, and high efficiency transformation protocols has made progress slow, but efforts in the past decade have led to the development of cloning vectors and transformation methods for these organisms. Some of the cloning vectors have broad host range (pRL series) whereas others have limited host range (pMEA300 and pMEA100). The cloning vector pMEA300 has been completely sequenced, while only the minimal replicon (pA-rep) has been sequenced from pRL plasmids. Direct transformation of mycelia and electroporation are the most widely applicable methods for transforming species of Amycolatopsis. Conjugational transfer from Escherichia coli has been reported only in the species A. japonicum, and gene disruption and replacements using homologous recombination are now possible in some strains.






Similar content being viewed by others
References
Alves AMCR, Meijer WG, Vrijbloed JW, Dijkhuizen L (1996) Characterization and phylogeny of the pfp gene of Amycolatopsis methanolica encoding pyrophosphate dependent phosphofructokinase. J Bacteriol 178:149–155
August, PR, Tang, L, Yoon, YJ, Ning, S, Muller, R, Yu, TW, Taylor M, Hoffman D, Kim G, Zhang X, Hutchinson CR, Floss HG (1998) Biosynthesis of the ansamycin antibiotic rifamycin: deductions from the molecular analysis of the rif biosynthetic gene cluster for Amycolatopsis mediterranei S699. Chem Biol 5:69–79
Baltz RH, Matsushima P (1983) Advances in protoplast fusion and transformation in Streptomyces. Exper Suppl 46:143–148
Barna JC, Williams DH (1984) The structure and mode of action of glycopeptide antibiotics of the vancomycin group. Annu Rev Microbiol 38:339–357
Bibb MJ, Ward JM, Hopwood DA (1978) Transformation of plasmid DNA into Streptomyces at high frequency. Nature 274:398–400
Biermann M, Logan R, O'Brien K, Seno ET, Nagaraja R, Schoner BE (1992) Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces sp. Gene 116:43–49
Chater KF, Hopwood DA, Kieser T, Thompson CJ (1982) Gene cloning in Streptomyces. Curr Topics Microbiol Immunol 96:69–95
De Boer L, Vrijbloed JW, Grobben G, Dijkhuizen, L (1989) Regulation of aromatic amino acid biosynthesis in the ribulose monophosphate cycle methylotroph Nocardia sp. 239. Arch Microbiol 152:319–325
Dijkhuizen L, Levering PR, De Vries GE (1993) The physiology and biochemistry of aerobic methanol-utilizing gram-negative and gram-positive bacteria. In: Atkinson T, Sherwood, RF (eds) Biotechnology handbooks. Methane and methanol-utilizers. Plenum , New York, pp149–181
Goodfellow M, Brown AB, Cai J, Chun J, Collins MD (1997) Amycolatopsis japonicum sp.nov., an actinomycete producing (S,S)-N,N′-ethylenediaminedisuccinic acid. Syst Appl Microbio 20:78–84
Grund EC, Knorr C, Eichenlaub R (1990) Catabolism of benzoate and monohydroxylated benzoates by Amycolatopsis and Streptomyces spp. Appl Environ Microbiol 56:1459–1464
Hektor HJ, Dijkhuzien L (1996) Mutational analysis of primary alcohol metabolism in the methylotrophic actinomycete Amycolatopsis methanolica. FEMS Microbiol Lett 144:73–79
Hu Z, Huuzikler D, Hutchinson CR, Khosla C (1999) A host vector system for analysis and manipulation of rifamycin polyketide biosynthesis in Amycolatopsis mediterranei. Microbiology 145:2335–2341
Ikuta N, Souza MB, Valencia FF, Castro ME, Schenberg AC, Pizzirani-Kleiner A, Astolfi-Filho S (1999) The α-amylase gene as a marker for gene cloning: direct screening of recombinant clones. Biotechnology 8:241–242
Kaur H, Cortes J, Leadlay P, Lal R (2001) Cloning and partial characterization of the presumptive rifamycin biosynthetic gene cluster from the actinomycete Amycolatopsis mediterranei DSM 46095. Microbiological Res 156:1–8
Khanna M, Dua M, Lal R (1998) Selection of suitable marker genes for the development of cloning vectors and electroporation in different strains of A. mediterranei. Microbiol Res 153:205–211
Kieser T, Bibb MJ, Buttner MJ, Chater KF Hopwood DA (1991) Practical Streptomyces genetics, a laboratory manual 2000. John Innes Foundation, Norwich
Kuhstoss S, Rao RN (1991) Analysis of the integration function of the streptomycete bacteriophage ϕC31. J Mol Biol 222:897–908
Kumar CV, Coque JJR, Martin JF (1994) Efficient transformation of the cephamycin C producer Nocardia lactamdurans and development of shuttle and promoter-probe cloning vectors. Appl Environ Microbiol 60:4086–4093
Kumari R, Bala S, Dhingra G, Majumdar S, Lal S, Cullum J, Lal R (2003) Optimization of transformation protocol and development of a system for homologous recombinations in Amycolatopsis. J Biotech 2003. Submitted
Lal R (1999) Cloning vector and a process for the preparation thereof. 1999 Patent US005985560A
Lal R, Khanna R, Dhingra N, Khanna M, Lal S (1998) Development of an improved cloning vector and transformation system in Amycolatopsis mediterranei. J Antibiot 51:161–169
Lal R, Kumari R, Kaur H, Khanna R, Dhingra N, Tuteja D (2000) Regulation of type I PKS gene clusters and their manipulations. Trends Biotechnol 18:264–274
Lal R, Lal S, Grund E, Eichenlaub R (1991) Construction of a hybrid plasmid capable of replication in Amycolatopsis mediterranei. Appl Environ Microbiol 57:665–671
Lechevalier MP, Prauser H, Labeda DP, Ruan J-S (1986) Two new genera of nocardioform actinomycetes: Amycolata gen. nov. and Amycolatopsis gen. nov. Int J Syst Bacteriol 36: 29–37
Madoń J, Moretti P, Hütter R (1987) Site specific integration and excision of pMEA100 in Nocardia mediterranei. Mol Gen Genet 209: 257–264
Madon J, Hütter R (1991) Transformation system for Amycolatopsis mediterranei: Direct transformation of mycelium with plasmid DNA. J Bacteriol 173: 6325–6331
Martin JF (1995) A method of transformation of Nocardia. Patent WO 95/23844
Matsumoto N, Tsuchida T, Sawa R, Iinuma H, Nakamura H, Naganawa H, Sawa T, Takeuchi T (1997) Epoxyquinomicins A, B, C and D, new antibiotics from Amycolatopsis. III. Physicochemical properties and structure determination. J. Antibiot 50:912–915
Matsushima P, Baltz RH (1985) Efficient plasmid transformation of Streptomyces ambofaciens and Streptomyces fradiae protoplasts. J Bacteriol 163:180–185.
Matsushima P, McHenney MA, Baltz RH (1987) Efficient transformation of Amycolatopsis orientalis (Nocardia orientalis) protoplasts by streptomyces plasmids. J Bacteriol 169, 2298–2300
Mazodier P, Thompson C, Boccard F (1990) The chromosomal integration site of the Streptomyces element pSAM2 overlaps a putative tRNA gene conserved among actinomycetes. Mol Gen Genet 222:431–434
Moretti P, Hintermann G, Hütter R (1985) Isolation and characterization of an extrachromosomal element from Nocardia mediterranei. Plasmid14:126–133
Nadkarni SR, Patel MV, Chatterjee S, Vijay Kumar EKS, Desikan KR, Bhimbach J, Ganguli BN (1994) Balhimycin, a new glycopeptide antibitotic produced by Amycolatopsis sp. Y–86 21022. J Antibiot 47:334–341
Pelzer S, Reichert W, Huppert M, Heckmann D, Wohlleben W (1997) Cloning and analysis of a peptide synthetase gene of the balhimycin producer Amycolatopsis mediterranei DSM 5908 and development of gene disruption/replacement system. J Biotechnol 56:115–128
Priefert H, Achterholt S, Steinbuchel A (2002) Transformation of the Pseudonocardiaceae Amycolatopsis sp. strain HR167 is highly dependent on the physiological state of the cells. Appl Microbiol Biotechnol 58:454–460
Schupp T, Divers M (1986) Protoplast preparation and regeneration in A. mediterranei. FEMS Microbiol Lett 36:159–162
Sensi P, Margalith P, Timbal MT (1959) Rifamycin, a new antibiotic: preliminary report. Farmaco Ed Sci 14:146–147
Shimanaka K, Kinoshita N, Iinuma H, Hamada M, Takeuchi T (1994) Novel antibiotics, amythiamycins. I. Taxonomy, fermentation, isolation, physico–chemical properties and antimicrobial activity. J Antibiot 47:668–674
Stegmann E, Pelzer S, Wilken K, Wohlleben W (2001) Development of three different gene cloning systems for genetic investigation of the new species Amycolatopsis japonicum MG417–CF17, the ethylenediaminedisuccinic acid (EDDS) producer. J Biotechnol 92:195–204
Thompson CJ, Ward JM, Hopwood DA (1982) Cloning of antibiotic resistance and nutritional genes in Streptomyces. J Bacteriol 151:668–677
Tsuchida T, Iinuma H, Kinoshita N, Ikeda T, Sawa R, Takahashi Y, Naganawa H, Sawa T, Hamada M, Takeuchi T (1993) Azicemicin A, a new antimicrobial antibiotic from Amycolatopsis. J Antibiot:46:1772–1774
Tsuchida T, Sawa R, Takahashi Y, Iinuma H, Sawa T, Naganawa H, Takeuchi T (1995) Azicemicins A and B, new antimicrobial agents produced by Amycolatopsis. II. Structure determination. J Antibiot 48:1148–1152
Tsuchida T, Umekita M, Kinoshita N, Iinuma H, Nakamura H, Nakamura K, Naganawa H, Sawa T, Hamada M, Takeuchi T(1996) Epoxyquinomicins A and B, new antibiotics from Amycolatopsis. J Antibiot 49:326–328
Tuteja D, Dua M, Khanna R, Dhingra N, Khanna M, Kaur H, Saxena DM, Lal R (2000) The importance of homologous recombination in the generation of large deletions in hybrid plasmids in A. mediterranei. Plasmid 3:1–11
Vrijbloed, J.W (1996) Functional analysis of the integrative plasmid pMEA 300 of the actinomycete Amycolatopsis methanolica. Ph.D thesis, Department of Microbiology, University of Groningen, Netherlands
Vrijbloed JW, Hylckama VJ, Put van der NMJ, Hessels GI, Dijkhuizen L (1995c) Molecular cloning with a pMEA300 derived shuttle vector and characterization of the Amycolatopsis methanolica prephenate dehydratase gene. J Bacteriol 177:6666–6669
Vrijbloed JW, Jelinkova M, Hessels GI, Dijkhuizen L (1995b) Identification of minimal replicon of plasmid pMEA300 of the methylotrophic actinomycete Amycolatopsis methanolica. Mol Microbiol 18:21–31
Vrijbloed JW, Madoń J, Dijkhuizen L (1994) A plasmid from the methylotrophic actinomycete Amycolatopsis methanolica capable of site-specific integration. J Bacteriol 176:7087–7090
Vrijbloed JW, Madoń J, Dijkhuizen L (1995a) Transformation of the methylotrophic actinomycete Amycolatopsis methanolica with plasmid DNA: stimulatory effect of a pMEA300 encoded gene. Plasmid 34:96–104
Vujaklija D, Davies J (1995) Direct transfer of bacterial plasmid DNA between Streptomyces spp. and E. coli by electroduction. J Antibiot 48:635–637
Wageningen AM van, Kirkpatric PN, Williams DH, Harris BR, Kershaw JK, Lennard NJ, Jones M, Jones SJ, Solenberg PJ (1998) Sequencing and analysis of genes involved in the biosynthesis of vancomycin group antibiotic. Chem Biol 5:155–162
Wang NJ, Han BL, Yameshita N, Sato M (1994) 31-Homorifamycin W, a novel metabolite from Amycolatopsis mediterranei. J Antibiot 47:613–615
Yamamoto H, Maures KH, Hutchinson CR (1986) Transformation of Streptomyces erythraeus. J Antibiot 39:1304–1313
Yao W, Yang Y, Chiao J (1994) Cloning vector system for Nocardia spp. Curr Microbiol 29:223–227
Zhu B, Madoń J, Häusler A, Hütter R (1990) Amplification on the Amycolatopsis (Nocardia) mediteranei plasmid pMEA100: sequence similarities to actinomycete att sites. Plasmid 24:132–142
Acknowledgements
The project was supported in part by grants from the Department of Science and Technology, Government of India. R. Kumari, Swati Majumdar, Shweta Malhotra and Poonam Sharma gratefully acknowledge the Council of Scientific and Industrial Research (CSIR), and the University Grants Commission (UGC), respectively, for providing doctoral fellowships. Rup Lal and John Cullum thank the Department of Science and Technology (Govt. of India) and the Deutsche Akademische Austauschdienst (DAAD), Germany, for support under the project-based personnel exchange programme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhingra, G., Kumari, R., Bala, S. et al. Development of cloning vectors and transformation methods for Amycolatopsis . J IND MICROBIOL BIOTECHNOL 30, 195–204 (2003). https://doi.org/10.1007/s10295-003-0040-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-003-0040-6