Abstract
Purpose
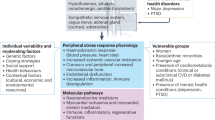
Mental stress is of essential consideration when assessing cardiovascular pathophysiology in all patient populations. Substantial evidence indicates associations among stress, cardiovascular disease and aberrant brain–body communication. However, our understanding of the flow of stress information in humans, is limited, despite the crucial insights this area may offer into future therapeutic targets for clinical intervention.
Methods
Key terms including mental stress, cardiovascular disease and central control, were searched in PubMed, ScienceDirect and Scopus databases. Articles indicative of heart rate and blood pressure regulation, or central control of cardiovascular disease through direct neural innervation of the cardiac, splanchnic and vascular regions were included. Focus on human neuroimaging research and the flow of stress information is described, before brain–body connectivity, via pre-motor brainstem intermediates is discussed. Lastly, we review current understandings of pathophysiological stress and cardiovascular disease aetiology.
Results
Structural and functional changes to corticolimbic circuitry encode stress information, integrated by the hypothalamus and amygdala. Pre-autonomic brain–body relays to brainstem and spinal cord nuclei establish dysautonomia and lead to alterations in baroreflex functioning, firing of the sympathetic fibres, cellular reuptake of norepinephrine and withdrawal of the parasympathetic reflex. The combined result is profoundly adrenergic and increases the likelihood of cardiac myopathy, arrhythmogenesis, coronary ischaemia, hypertension and the overall risk of future sudden stress-induced heart failure.
Conclusions
There is undeniable support that mental stress contributes to the development of cardiovascular disease. The emerging accumulation of large-scale multimodal neuroimaging data analytics to assess this relationship promises exciting novel therapeutic targets for future cardiovascular disease detection and prevention.


Similar content being viewed by others
References
Gianaros PJ, Wager TD (2015) Brain-body pathways linking psychological stress and physical health. Curr Dir Psychol Sci 24(4):313–321. https://doi.org/10.1177/0963721415581476
Henein MY, Vancheri S, Longo G, Vancheri F (2022) The impact of mental stress on cardiovascular health—part II. J Clin Med 11(15):4405. https://doi.org/10.3390/jcm11154405
Liu M-Y, Li N, Li WA, Khan H (2017) Association between psychosocial stress and hypertension: a systematic review and meta-analysis. Neurol Res 39(6):573–580. https://doi.org/10.1080/01616412.2017.1317904
Brunier A (2020) WHO reveals leading causes of death and disability worldwide. 2000–2019. (n.d.). https://www.who.int/news/item/09-12-2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000-2019. 3 Apr 2023
Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, Grandner MA, Lavretsky H, Perak AM, Sharma G, Rosamond W, Association AH (2022) Life’s essential 8: updating and enhancing the american heart association’s construct of cardiovascular health: a presidential advisory from the american heart association. Circulation 146(5):e18–e43. https://doi.org/10.1161/CIR.0000000000001078
Cintenza M (2021) How do we measure stress as a risk factor? Mædica 16(1):3–5. https://doi.org/10.26574/maedica.2020.16.1.3
Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L (2004) Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. The Lancet 364(9438):937–952. https://doi.org/10.1016/S0140-6736(04)17018-9
Eddy P, Wertheim EH, Kingsley M, Wright BJ (2017) Associations between the effort-reward imbalance model of workplace stress and indices of cardiovascular health: a systematic review and meta-analysis. Neurosci Biobehav Rev 83:252–266. https://doi.org/10.1016/j.neubiorev.2017.10.025
Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D (2015) Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci 10(2):227–237. https://doi.org/10.1177/1745691614568352
Mira JJ, Carrillo I, Guilabert M, Mula A, Martin-Delgado J, Pérez-Jover MV, Vicente MA, Fernández C (2020) Acute stress of the healthcare workforce during the COVID-19 pandemic evolution: a cross-sectional study in Spain. BMJ Open 10(11):e042555. https://doi.org/10.1136/bmjopen-2020-042555
Marin M-F, Geoffrion S, Juster R-P, Giguère C-E, Marchand A, Lupien SJ, Guay S (2019) High cortisol awakening response in the aftermath of workplace violence exposure moderates the association between acute stress disorder symptoms and PTSD symptoms. Psychoneuroendocrinology 104:238–242. https://doi.org/10.1016/j.psyneuen.2019.03.006
Appiah D, Noamesi AT, Osaji J, Bolton C, Nwabuo CC, Ebong IA (2022) The association of mental health disorders with takotsubo syndrome (broken heart syndrome) among older women. Journal of Women’s Health 31(9):1334–1342. https://doi.org/10.1089/jwh.2021.0557
Chandola T, Britton A, Brunner E, Hemingway H, Malik M, Kumari M, Badrick E, Kivimaki M, Marmot M (2008) Work stress and coronary heart disease: what are the mechanisms? Eur Heart J 29(5):640–648. https://doi.org/10.1093/eurheartj/ehm584
Chauvet-Gelinier J-C, Bonin B (2017) Stress, anxiety and depression in heart disease patients: a major challenge for cardiac rehabilitation. Ann Phys Rehabil Med 60(1):6–12. https://doi.org/10.1016/j.rehab.2016.09.002
Kilbourn KJ, Levy S, Staff I, Kureshi I, McCullough L (2013) Clinical characteristics and outcomes of neurogenic stress aphemyopathy in aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg 115(7):909–914. https://doi.org/10.1016/j.clineuro.2012.09.006
Maeng LY, Milad MR (2017) Post-traumatic stress disorder: The relationship between the fear response and chronic stress. Chronic Stress 1:2470547017713297. https://doi.org/10.1177/2470547017713297
Cannon WB (1927) The James-Lange theory of emotions: a critical examination and an alternative theory. Am J Psychol 39(1/4):106–124. https://doi.org/10.2307/1415404
Lang PJ (1994) The varieties of emotional experience: a meditation on James-Lange theory. Psychol Review 101(2):211–221. https://doi.org/10.1037/0033-295X.101.2.211
Selye H (1936) A Syndrome produced by diverse nocuous agents. Nature. https://doi.org/10.1038/138032a0
Kvetnansky R (2004) Stressor specificity and effect of prior experience on catecholamine biosynthetic enzyme phenylethanolamine N-methyltransferase. Ann N Y Acad Sci 1032:117–129. https://doi.org/10.1196/annals.1314.009. (PMID: 15677399)
Friedman M, Rosenman RH (1959) Association of specific overt behaviour pattern with blood and cardiovascular findings: blood cholesterol level, blood clotting time, incidence of arcus senilis, and clinical coronary artery disease. J Am Med Assoc 169(12):1286–1296. https://doi.org/10.1001/jama.1959.03000290012005
Chrousos GP, Gold PW (1992) The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 267(9):1244–1252. https://doi.org/10.1001/jama.1992.03480090092034
Benarroch EE (1993) The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc 68(10):988–1001. https://doi.org/10.1016/S0025-6196(12)62272-1
Macefield VG, Henderson LA (2019) Identification of the human sympathetic connectome involved in blood pressure regulation. Neuroimage 202:116119. https://doi.org/10.1016/j.neuroimage.2019.116119
Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ (2004) Neural systems supporting interoceptive awareness. Nat Neurosci. https://doi.org/10.1038/nn1176
Langley JN (1903) The autonomic nervous sytem. Brain 26(1):1–26. https://doi.org/10.1093/brain/26.1.1
Goldstein DS (2021) Stress and the “extended” autonomic system. Auton Neurosci 236:102889. https://doi.org/10.1016/j.autneu.2021.102889
Feigofsky S, Fedorowski A (2020) Defining cardiac dysautonomia–different types, overlap syndromes Case-based presentations. J Atrial Fibrillation 13(1):2403. https://doi.org/10.4022/jafib.2403
Donadio V, Liguori R, Elam M, Karlsson T, Giannoccaro MP, Pegenius G, Giambattistelli F, Wallin BG (2012) Muscle sympathetic response to arousal predicts neurovascular reactivity during mental stress. J Physiol 590(12):2885–2896. https://doi.org/10.1113/jphysiol.2012.228981
El Sayed K, Macefield VG, Hissen SL, Joyner MJ, Taylor CE (2016) Rate of rise in diastolic blood pressure influences vascular sympathetic response to mental stress. J Physiol 594(24):7465–7482. https://doi.org/10.1113/JP272963
Jiang R, Calhoun VD, Noble S, Sui J, Liang Q, Qi S, Scheinost D (2022) A functional connectome signature of blood pressure in >30 000 participants from the UK biobank. Cardiovasc Res. https://doi.org/10.1093/cvr/cvac116
Dichtl W, Tuovinen N, Barbieri F, Adukauskaite A, Senoner T, Rubatscher A, Hintringer F, Siedentopf C, Bauer A, Gizewski ER, Steiger R (2020) Functional neuroimaging in the acute phase of Takotsubo syndrome: volumetric and functional changes of the right insular cortex. Clin Res Cardiol 109(9):1107–1113. https://doi.org/10.1007/s00392-020-01602-3
Zhang J, Richardson JD, Dunkley BT (2020) Classifying post-traumatic stress disorder using the magnetoencephalographic connectome and machine learning. Sci Rep 10:5937. https://doi.org/10.1038/s41598-020-62713-5
Kresoja K-P, Unterhuber M, Wachter R, Thiele H, Lurz P (2023) A cardiologist’s guide to machine learning in cardiovascular disease prognosis prediction. Basic Res Cardiol 118(1):10. https://doi.org/10.1007/s00395-023-00982-7
Mathur P, Srivastava S, Xu X, Mehta JL (2020) Artificial intelligence, machine learning, and cardiovascular disease. Clin Med Insights Cardiology 14:1179546820927404. https://doi.org/10.1177/1179546820927404
Andersen NH, Jørgensen T, Brodersen JB (2023) More costs than benefits from screening for cardiovascular disease with computed tomography in the DANCAVAS study. Eur Heart J 44(1):68–69. https://doi.org/10.1093/eurheartj/ehac634
Rossini PM, Di Iorio R, Bentivoglio M, Bertini G, Ferreri F, Gerloff C, Ilmoniemi RJ, Miraglia F, Nitsche MA, Pestilli F, Rosanova M, Shirota Y, Tesoriero C, Ugawa Y, Vecchio F, Ziemann U, Hallett M (2019) Methods for analysis of brain connectivity: an IFCN-sponsored review. Clin Neurophysiol 130(10):1833–1858. https://doi.org/10.1016/j.clinph.2019.06.006
Lampert R, Joska T, Burg MM, Batsford WP, McPherson CA, Jain D (2002) Emotional and physical precipitants of ventricular arrhythmia. Circulation 106(14):1800–1805. https://doi.org/10.1161/01.CIR.0000031733.51374.C1
Schultchen D, Bayer J, Kühnel J, Melchers KG, Pollatos O (2019) Interoceptive accuracy is related to long-term stress via self-regulation. Psychophysiology 56(10):e13429. https://doi.org/10.1111/psyp.13429
Williamson TJ, Thomas KS, Eisenberger NI, Stanton AL (2018) Effects of social exclusion on cardiovascular and affective reactivity to a socially evaluative stressor. Int J Behav Med 25(4):410–420. https://doi.org/10.1007/s12529-018-9720-5
McCarty R (2016) Chapter 4—the fight-or-flight response: a cornerstone of stress research. In: Fink G (ed) Stress: concepts, cognition, emotion, and behavior. Academic Press, Cham, pp 33–37
Tanev KS, Orr SP, Pace-Schott EF, Griffin M, Pitman RK, Resick PA (2017) Positive association between nightmares and heart rate response to loud tones: relationship to parasympathetic dysfunction in PTSD nightmares. J Nerv Ment Dis 205(4):308–312. https://doi.org/10.1097/NMD.0000000000000641
Parmar MS, Luque-Coqui AF (1998) Killer dreams. Can J Cardiol 14(11):1389–1391
Frick A, Björkstrand J, Lubberink M, Eriksson A, Fredrikson M, Åhs F (2022) Dopamine and fear memory formation in the human amygdala. Mol Psychiatry 27(3):1704–1711. https://doi.org/10.1038/s41380-021-01400-x
Tang W, Kochubey O, Kintscher M, Schneggenburger R (2020) A VTA to basal amygdala dopamine projection contributes to signal salient somatosensory events during fear learning. J Neurosci 40(20):3969–3980. https://doi.org/10.1523/JNEUROSCI.1796-19.2020
Fudge JL, Tucker T (2009) Amygdala projections to central amygdaloid nucleus subdivisions and transition zones in the primate. Neuroscience 159(2):819–841. https://doi.org/10.1016/j.neuroscience.2009.01.013
Dayas CV, Buller KM, Crane JW, Xu Y, Day TA (2001) Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci 14(7):1143–1152. https://doi.org/10.1046/j.0953-816x.2001.01733.x
McDougall SJ, Widdop RE, Lawrence AJ (2005) Central autonomic integration of psychological stressors: focus on cardiovascular modulation. Auton Neurosci: Basic Clin 123(1):1–11. https://doi.org/10.1016/j.autneu.2005.09.005
Fang F, Arnberg FK, Mataix-Cols D, Fernández de la Cruz L, Almqvist C, Fall K, Lichtenstein P, Thorgeirsson G, Valdimarsdóttir UA, Song H (2019) Stress related disorders and risk of cardiovascular disease: population based, sibling controlled cohort study. The BMJ 365:l1255. https://doi.org/10.1136/bmj.l1255
Esler M, Jennings G, Lambert G (1989) Measurement of overall and cardiac norepinephrine release into plasma during cognitive challenge. Psychoneuroendocrinology 14(6):477–481. https://doi.org/10.1016/0306-4530(89)90047-4
Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Lüscher TF (2015) Clinical features and outcomes of takotsubo (Stress) cardiomyopathy. N Engl J Med 373(10):929–938. https://doi.org/10.1056/NEJMoa1406761
Barton DA, Dawood T, Lambert EA, Esler MD, Haikerwal D, Brenchley C, Socratous F, Kaye DM, Schlaich MP, Hickie I, Lambert GW (2007) Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J Hypertens 25(10):2117–2124. https://doi.org/10.1097/HJH.0b013e32829baae7
Kiecolt-Glaser JK, Derry HM, Fagundes CP (2015) Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry 172(11):1075–1091. https://doi.org/10.1176/appi.ajp.2015.15020152
Baumert M, Lambert GW, Dawood T, Lambert EA, Esler MD, McGrane M, Barton D, Nalivaiko E (2008) QT interval variability and cardiac norepinephrine spillover in patients with depression and panic disorder. Am J Physiol-Heart Circ Physiol 295(3):H962–H968. https://doi.org/10.1152/ajpheart.00301.2008
Meijer A, Conradi HJ, Bos EH, Thombs BD, van Melle JP, de Jonge P (2011) Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis of 25 years of research. Gen Hosp Psychiatry 33(3):203–216. https://doi.org/10.1016/j.genhosppsych.2011.02.007
Rosenkranz JA, Venheim ER, Padival M (2010) Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiat 67(12):1128–1136. https://doi.org/10.1016/j.biopsych.2010.02.008
Lee S-C, Amir A, Haufler D, Pare D (2017) Differential recruitment of competing valence-related amygdala networks during anxiety. Neuron 96(1):81-88.e5. https://doi.org/10.1016/j.neuron.2017.09.002
McKlveen JM, Myers B, Herman JP (2015) The medial prefrontal cortex: coordinator of autonomic, neuroendocrine, and behavioral responses to stress. J Neuroendocrinol 27(6):446–456. https://doi.org/10.1111/jne.12272
Pace SA, Christensen C, Schackmuth MK, Wallace T, McKlveen JM, Beischel W, Morano R, Scheimann JR, Wilson SP, Herman JP, Myers B (2020) Infralimbic cortical glutamate output is necessary for the neural and behavioral consequences of chronic stress. Neurobiol Stress 13:100274. https://doi.org/10.1016/j.ynstr.2020.100274
Schaeuble D, Packard AEB, McKlveen JM, Morano R, Fourman S, Smith BL, Scheimann JR, Packard BA, Wilson SP, James J, Hui DY, Ulrich-Lai YM, Herman JP, Myers B (2019) Prefrontal cortex regulates chronic stress-induced cardiovascular susceptibility. J Am Heart Assoc: Cardiovasc Cerebrovasc Dis 8(24):e014451. https://doi.org/10.1161/JAHA.119.014451
Swanson LW, Kuypers HGJM (1980) The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194(3):555–570. https://doi.org/10.1002/cne.901940306
Ferreira-Junior NC, Fedoce AG, Alves FHF, Resstel LBM (2013) Medial prefrontal cortex N-methyl-D-aspartate receptor/nitric oxide/cyclic guanosine monophosphate pathway modulates both tachycardic and bradycardic baroreflex responses. J Neurosci Res 91(10):1338–1348. https://doi.org/10.1002/jnr.23248
Lagatta DC, Kuntze LB, Ferreira-Junior NC, Resstel LBM (2018) Medial prefrontal cortex TRPV1 and CB1 receptors modulate cardiac baroreflex activity by regulating the NMDA receptor/nitric oxide pathway. Pflügers Archiv—Eur J Physiol 470(10):1521–1542. https://doi.org/10.1007/s00424-018-2149-5
Rodriguez-Miguelez P, Looney J, Blackburn M, Thomas J, Pollock JS, Harris RA (2022) The link between childhood adversity and cardiovascular disease risk: role of cerebral and systemic vasculature. Function 3(4):zqac029. https://doi.org/10.1093/function/zqac029
Moazzami K, Wittbrodt MT, Lima BB, Nye JA, Mehta PK, Pearce BD, Almuwaqqat Z, Hammadah M, Levantsevych O, Sun YV, Raggi P, Garcia EV, Goetz M, Quyyumi AA, Bremner JD, Vaccarino V, Shah AJ (2020) Higher activation of the rostromedial prefrontal cortex during mental stress predicts major cardiovascular disease events in individuals with coronary artery disease. Circulation 142(5):455–465. https://doi.org/10.1161/CIRCULATIONAHA.119.044442
Moazzami K, Wittbrodt MT, Alkhalaf M, Lima BB, Nye JA, Mehta PK, Quyyumi AA, Vaccarino V, Bremner JD, Shah AJ (2020) Association between mental stress-induced inferior frontal cortex activation and angina in coronary artery disease. Circ Cardiovasc Imaging 13(8):e010710. https://doi.org/10.1161/CIRCIMAGING.120.010710
Wittbrodt MT, Moazzami K, Shah AJ, Lima BB, Hammadah M, Mehta PK, Quyyumi AA, Vaccarino V, Nye JA, Bremner JD (2020) Neural responses during acute mental stress are associated with angina pectoris. J Psychosom Res 134:110110. https://doi.org/10.1016/j.jpsychores.2020.110110
Rosen SD, Paulesu E, Frith CD, Frackowiak RSJ, Davies GJ, Jones T, Camici PG (1994) Central nervous pathways mediating angina pectoris. The Lancet 344(8916):147–150. https://doi.org/10.1016/S0140-6736(94)92755-3
Buchanan TW, Driscoll D, Mowrer SM, Sollers JJ, Thayer JF, Kirschbaum C, Tranel D (2010) Medial prefrontal cortex damage affects physiological and psychological stress responses differently in men and women. Psychoneuroendocrinology 35(1):56–66. https://doi.org/10.1016/j.psyneuen.2009.09.006
Lupis SB, Lerman M, Wolf JM (2014) Anger responses to psychosocial stress predict heart rate and cortisol stress responses in men but not women. Psychoneuroendocrinology 49:84–95. https://doi.org/10.1016/j.psyneuen.2014.07.004
Bürger Z, Müller VI, Hoffstaedter F, Habel U, Gur RC, Windischberger C, Moser E, Derntl B, Kogler L (2023) Stressor-specific sex differences in amygdala–frontal cortex networks. J Clin Med. https://doi.org/10.3390/jcm12030865
Salinas-Hernández XI, Duvarci S (2021) Dopamine in fear extinction. Front Synaptic Neurosci 13:635879. https://doi.org/10.3389/fnsyn.2021.635879
Seligowski AV, Merker JB, Swiercz AP, Park J, Marvar PJ, Ressler KJ, Jovanovic T (2020) Examining the cardiovascular response to fear extinction in a trauma-exposed sample. J Psychiatr Res 124:85–90. https://doi.org/10.1016/j.jpsychires.2020.02.024
Mohanty A, Engels AS, Herrington JD, Heller W, Ringo Ho M-H, Banich MT, Webb AG, Warren SL, Miller GA (2007) Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology 44(3):343–351. https://doi.org/10.1111/j.1469-8986.2007.00515.x
Liberzon I, King AP, Britton JC, Phan KL, Abelson JL, Taylor SF (2007) Paralimbic and medial prefrontal cortical involvement in neuroendocrine responses to traumatic stimuli. Am J Psychiatry 164(8):1250–1258. https://doi.org/10.1176/appi.ajp.2007.06081367
King AP, Abelson JL, Britton JC, Phan KL, Taylor SF, Liberzon I (2009) Medial prefrontal cortex and right insula activity predict plasma ACTH response to trauma recall. Neuroimage 47(3):872–880. https://doi.org/10.1016/j.neuroimage.2009.05.088
Fee C, Prevot T, Misquitta K, Banasr M, Sibille E (2020) Chronic stress-induced behaviors correlate with exacerbated acute stress-induced cingulate cortex and ventral hippocampus activation. Neuroscience 440:113–129. https://doi.org/10.1016/j.neuroscience.2020.05.034
Joyce MKP, García-Cabezas MÁ, John YJ, Barbas H (2020) Serial prefrontal pathways are positioned to balance cognition and emotion in primates. J Neurosci 40(43):8306–8328. https://doi.org/10.1523/JNEUROSCI.0860-20.2020
Critchley HD, Harrison NA (2013) Visceral influences on brain and behavior. Neuron 77(4):624–638. https://doi.org/10.1016/j.neuron.2013.02.008
Sesa-Ashton G, Wong R, McCarthy B, Datta S, Henderson LA, Dawood T, Macefield VG (2022) Stimulation of the dorsolateral prefrontal cortex modulates muscle sympathetic nerve activity and blood pressure in humans. Cerebral Cortex Commun 3(2):017. https://doi.org/10.1093/texcom/tgac017
Riaz B, Eskelin JJ, Lundblad LC, Wallin BG, Karlsson T, Starck G, Lundqvist D, Oostenveld R, Schneiderman JF, Elam M (2022) Brain structural and functional correlates to defense-related inhibition of muscle sympathetic nerve activity in man. Sci Rep 12:1990. https://doi.org/10.1038/s41598-022-05910-8
Jezzini A, Rozzi S, Borra E, Gallese V, Caruana F, Gerbella M (2015) A shared neural network for emotional expression and perception: an anatomical study in the macaque monkey. Front Behav Neurosci 9:243. https://doi.org/10.3389/fnbeh.2015.00243
Ruiz Vargas E, Sörös P, Shoemaker JK, Hachinski V (2016) Human cerebral circuitry related to cardiac control: a neuroimaging meta-analysis. Ann Neurol 79(5):709–716. https://doi.org/10.1002/ana.24642
Terasawa Y, Kurosaki Y, Ibata Y, Moriguchi Y, Umeda S (2015) Attenuated sensitivity to the emotions of others by insular lesion. Front Psychol 6:1314. https://doi.org/10.3389/fpsyg.2015.01314
Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC (1992) Cardiovascular effects of human insular cortex stimulation. Neurology 42(9):1727–1727. https://doi.org/10.1212/WNL.42.9.1727
Chouchou F, Mauguière F, Vallayer O, Catenoix H, Isnard J, Montavont A, Jung J, Pichot V, Rheims S, Mazzola L (2019) How the insula speaks to the heart: cardiac responses to insular stimulation in humans. Hum Brain Mapp 40(9):2611–2622. https://doi.org/10.1002/hbm.24548
Henderson LA, Macey PM, Macey KE, Frysinger RC, Woo MA, Harper RK, Alger JR, Yan-Go FL, Harper RM (2002) Brain responses associated with the valsalva maneuver revealed by functional magnetic resonance imaging. J Neurophysiol 88(6):3477–3486. https://doi.org/10.1152/jn.00107.2002
Harper RM, Macey PM, Henderson LA, Woo MA, Macey KE, Frysinger RC, Alger JR, Nguyen KP, Yan-Go FL (2003) FMRI responses to cold pressor challenges in control and obstructive sleep apnea subjects. J Appl Physiol 94(4):1583
Macefield VG, Gandevia SC, Henderson LA (2006) Neural sites involved in the sustained increase in muscle sympathetic nerve activity induced by inspiratory capacity apnea: a fMRI study. J Appl Physiol 100(1):266–273. https://doi.org/10.1152/japplphysiol.00588.2005
Wong SW, Massé N, Kimmerly DS, Menon RS, Shoemaker JK (2007) Ventral medial prefrontal cortex and cardiovagal control in conscious humans. Neuroimage 35(2):698–708. https://doi.org/10.1016/j.neuroimage.2006.12.027
Kimmerly DS, O’Leary DD, Menon RS, Gati JS, Shoemaker JK (2005) Cortical regions associated with autonomic cardiovascular regulation during lower body negative pressure in humans. J Physiol 569(Pt 1):331–345. https://doi.org/10.1113/jphysiol.2005.091637
Macey PM, Rieken NS, Ogren JA, Macey KE, Kumar R, Harper RM (2017) Sex differences in insular cortex gyri responses to a brief static handgrip challenge. Biol Sex Differ 8(1):13. https://doi.org/10.1186/s13293-017-0135-9
Kimmerly DS, Wong S, Menon R, Shoemaker JK (2007) Forebrain neural patterns associated with sex differences in autonomic and cardiovascular function during baroreceptor unloading. Am J Physiol-Regul, Integr Comp Physiol 292(2):R715–R722. https://doi.org/10.1152/ajpregu.00366.2006
Groenland EH, van Kleef MEAM, Hendrikse J, Spiering W, Siero JCW (2022) The effect of endovascular baroreflex amplification on central sympathetic nerve circuits and cerebral blood flow in patients with resistant hypertension: a functional MRI study. Front Neuroimaging. https://doi.org/10.3389/fnimg.2022.924724
Lacuey N, Hampson JP, Theeranaew W, Zonjy B, Vithala A, Hupp NJ, Loparo KA, Miller JP, Lhatoo SD (2018) Cortical structures associated with human blood pressure control. JAMA Neurol 75(2):194–202. https://doi.org/10.1001/jamaneurol.2017.3344
Fonzo GA, Goodkind MS, Oathes DJ, Zaiko YV, Harvey M, Peng KK, Weiss ME, Thompson AL, Zack SE, Lindley SE, Arnow BA, Jo B, Rothbaum BO, Etkin A (2021) Amygdala and insula connectivity changes following psychotherapy for posttraumatic stress disorder: a randomized clinical trial. Biol Psychiat 89(9):857–867. https://doi.org/10.1016/j.biopsych.2020.11.021
Zhu Y, Wang Y, Yang Z, Wang L, Hu X (2020) Endogenous cortisol-related alterations of right anterior insula functional connectivity under acute stress. J Affect Disord 274:231–238. https://doi.org/10.1016/j.jad.2020.05.123
Yu X, Cohen ZP, Tsuchiyagaito A, Cochran G, Aupperle RL, Stewart JL, Singh MK, Misaki M, Bodurka J, Paulus MP, Kirlic N (2022) Neurofeedback-augmented mindfulness training elicits distinct responses in the subregions of the insular cortex in healthy adolescents. Brain Sci 12(3):363. https://doi.org/10.3390/brainsci12030363
Buijs RM, Van Eden CG (2000) The integration of stress by the hypothalamus, amygdala and prefrontal cortex: balance between the autonomic nervous system and the neuroendocrine system. Progress in brain research, vol 126. Elsevier, pp 117–132
Kullmann S, Veit R (2021) Chapter 7—Resting-state functional connectivity of the human hypothalamus. In: Swaab DF, Kreier F, Lucassen PJ, Salehi A, Buijs RM (eds) Handbook of clinical neurology, vol 179. Elsevier, pp 113–124
Bear MH, Reddy V, Bollu PC (2022) Neuroanatomy, hypothalamus. StatPearl. StatPearls Publishing
Dampney RA (1994) Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. https://doi.org/10.1152/physrev.1994.74.2.323
Kinsman BJ, Simmonds SS, Browning KN, Stocker SD (2017) The organum vasculosum of the lamina terminalis detects NaCl to elevate sympathetic nerve activity and blood pressure. Hypertension (Dallas, Tex 1979) 69(1):163–170. https://doi.org/10.1161/HYPERTENSIONAHA.116.08372
Smith PM, Ferguson AV (2010) Circulating signals as critical regulators of autonomic state—central roles for the subfornical organ. Am J Physiol-Regul, Integr Comp Physiol 299(2):R405–R415. https://doi.org/10.1152/ajpregu.00103.2010
Zhang F, Sun H-J, Xiong X-Q, Chen Q, Li Y-H, Kang Y-M, Wang J-J, Gao X-Y, Zhu G-Q (2014) Apelin-13 and APJ in paraventricular nucleus contribute to hypertension via sympathetic activation and vasopressin release in spontaneously hypertensive rats. Acta Physiol 212(1):17–27. https://doi.org/10.1111/apha.12342
Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A (2005) Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci 25(49):11489–11493. https://doi.org/10.1523/JNEUROSCI.3984-05.2005
Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M (2016) Intranasal oxytocin normalizes amygdala functional connectivity in posttraumatic stress disorder. Neuropsychopharmacology 41(8):2041–2051. https://doi.org/10.1038/npp.2016.1
Adameova A, Abdellatif Y, Dhalla NS (2009) Role of the excessive amounts of circulating catecholamines and glucocorticoids in stress-induced heart disease. Can J Physiol Pharmacol 87(7):493–514. https://doi.org/10.1139/Y09-042
Bhagat BD, Rao PS, Dhalla NS (1980) Role of catecholamines in the genesis of arrhythmias. Adv Myocardiol 2:117–132
Hamer M, Endrighi R, Venuraju SM, Lahiri A, Steptoe A (2012) Cortisol responses to mental stress and the progression of coronary artery calcification in healthy men and women. PLoS ONE 7(2):e31356. https://doi.org/10.1371/journal.pone.0031356
Hamer M, Steptoe A (2012) Cortisol responses to mental stress and incident hypertension in healthy men and women. J Clin Endocrinol Metab 97(1):E29–E34. https://doi.org/10.1210/jc.2011-2132
Soltis RP, Cook JC, Gregg AE, Stratton JM, Flickinger KA (1998) EAA receptors in the dorsomedial hypothalamic area mediate the cardiovascular response to activation of the amygdala. Am J Physiol-Regul, Integr Comp Physiol 275(2):R624–R631. https://doi.org/10.1152/ajpregu.1998.275.2.R624
Bondarenko E, Beig MI, Hodgson DM, Braga VA, Nalivaiko E (2015) Blockade of the dorsomedial hypothalamus and the perifornical area inhibits respiratory responses to arousing and stressful stimuli. Am J Physiol-Regul, Integr Comp Physiol 308(10):R816–R822. https://doi.org/10.1152/ajpregu.00415.2014
McDowall LM, Horiuchi J, Killinger S, Dampney RAL (2006) Modulation of the baroreceptor reflex by the dorsomedial hypothalamic nucleus and perifornical area. Am J Physiol-Regul, Integr Comp Physiol 290(4):R1020–R1026. https://doi.org/10.1152/ajpregu.00541.2005
Dampney RAL (2019) Chapter 28—Central mechanisms generating cardiovascular and respiratory responses to emotional stress. In: Fink G (ed) Stress: physiology, biochemistry, and pathology. Academic Press, pp 391–402
Kono Y, Yokota S, Fukushi I, Arima Y, Onimaru H, Okazaki S, Takeda K, Yazawa I, Yoshizawa M, Hasebe Y, Koizumi K, Pokorski M, Toda T, Sugita K, Okada Y (2020) Structural and functional connectivity from the dorsomedial hypothalamus to the ventral medulla as a chronological amplifier of sympathetic outflow. Sci Rep 10:13325. https://doi.org/10.1038/s41598-020-70234-4
Carrive P, Gorissen M (2008) Premotor sympathetic neurons of conditioned fear in the rat. Eur J Neurosci 28(3):428–446. https://doi.org/10.1111/j.1460-9568.2008.06351.x
González-García M, Carrillo-Franco L, Peinado-Aragonés CA, Díaz-Casares A, Gago B, López-González MV, Dawid-Milner MS (2023) Impact of the glutamatergic neurotransmission within the A5 region on the cardiorespiratory response evoked from the midbrain dlPAG. Pflugers Archiv 475(4):505–516. https://doi.org/10.1007/s00424-022-02777-6
Moraes GCA, Mendonça MM, Mourão AA, Graziani D, Pinto MCX, Ferreira PM, Pedrino GR, Fontes MAP, Oliveira-Lima OC, Xavier CH (2020) Ventromedial medullary pathway mediating cardiac responses evoked from periaqueductal gray. Auton Neurosci 228:102716. https://doi.org/10.1016/j.autneu.2020.102716
Bacon SJ, Zagon A, Smith AD (1990) Electron microscopic evidence of a monosynaptic pathway between cells in the caudal aphe nuclei and sympathetic preganglionic neurons in the rat spinal cord. Exp Brain Res 79(3):589–602. https://doi.org/10.1007/BF00229327
Samuels BC, Zaretsky DV, DiMicco JA (2002) Tachycardia evoked by disinhibition of the dorsomedial hypothalamus in rats is mediated through medullary raphe. J Physiol 538(3):941–946. https://doi.org/10.1113/jphysiol.2001.013302
Fontes MAP, Filho ML, Machado NLS, de Paula CA, Cordeiro LMS, Xavier CH, Marins FR, Henderson L, Macefield VG (2017) Asymmetric sympathetic output: the dorsomedial hypothalamus as a potential link between emotional stress and cardiac arrhythmias. Auton Neurosci: Basic Clin 207:22–27. https://doi.org/10.1016/j.autneu.2017.01.001
Li T-L, Chen JYS, Huang S-C, Dai Y-WE, Hwang L-L (2018) Cardiovascular pressor effects of orexins in the dorsomedial hypothalamus. Eur J Pharmacol 818:343–350. https://doi.org/10.1016/j.ejphar.2017.11.004
Soya S, Takahashi TM, McHugh TJ, Maejima T, Herlitze S, Abe M, Sakimura K, Sakurai T (2017) Orexin modulates behavioral fear expression through the locus coeruleus. Nat Commun 8:1606. https://doi.org/10.1038/s41467-017-01782-z
Yamashita A, Moriya S, Nishi R, Kaminosono J, Yamanaka A, Kuwaki T (2021) Aversive emotion rapidly activates orexin neurons and increases heart rate in freely moving mice. Mol Brain 14(1):104. https://doi.org/10.1186/s13041-021-00818-2
Olsen N, Furlong TM, Carrive P (2021) Behavioural and cardiovascular effects of orexin-A infused into the central amygdala under basal and fear conditions in rats. Behav Brain Res 415:113515. https://doi.org/10.1016/j.bbr.2021.113515
Shi S, Xu AG, Rui Y-Y, Zhang X, Romanski LM, Gothard KM, Roe AW (2021) Infrared neural stimulation with 7T fMRI: a rapid in vivo method for mapping cortical connections of primate amygdala. Neuroimage 231:117818. https://doi.org/10.1016/j.neuroimage.2021.117818
Pitkänen A, Amaral DG (1991) Demonstration of projections from the lateral nucleus to the basal nucleus of the amygdala: a PHA-L study in the monkey. Exp Brain Res 83(3):465–470. https://doi.org/10.1007/BF00229822
Rodrigues SM, Schafe GE, LeDoux JE (2004) Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron 44(1):75–91. https://doi.org/10.1016/j.neuron.2004.09.014
Cho YT, Ernst M, Fudge JL (2013) Cortico–amygdala–striatal circuits are organized as hierarchical subsystems through the primate amygdala. J Neurosci 33(35):14017–14030. https://doi.org/10.1523/JNEUROSCI.0170-13.2013
Surget A, Belzung C (2022) Adult hippocampal neurogenesis shapes adaptation and improves stress response: a mechanistic and integrative perspective. Mol Psychiatry 27(1):403–421. https://doi.org/10.1038/s41380-021-01136-8
Marek R, Sah P (2018) Neural circuits mediating fear learning and extinction. Adv neurobiol 21:35–48. https://doi.org/10.1007/978-3-319-94593-4_2
Sah P, Faber ESL, Lopez De Armentia M, Power J (2003) The amygdaloid complex: anatomy and physiology. Physiol Rev 83(3):803–834. https://doi.org/10.1152/physrev.00002.2003
Chen LW, Sun D, Davis SL, Haswell CC, Dennis EL, Swanson CA, Whelan CD, Gutman B, Jahanshad N, Iglesias JE, Thompson P, Wagner HR, Saemann P, LaBar KS, Morey RA (2018) Smaller hippocampal CA1 subfield volume in posttraumatic stress disorder. Depress Anxiety 35(11):1018–1029. https://doi.org/10.1002/da.22833
Morey RA, Clarke EK, Haswell CC, Phillips RD, Clausen AN, Mufford MS, Saygin Z, Brancu M, Beckham JC, Calhoun PS, Dedert E, Elbogen EB, Fairbank JA, Hurley RA, Kilts JD, Kimbrel NA, Kirby A, Marx CE, McDonald SD, LaBar KS (2020) Amygdala nuclei volume and shape in military veterans with posttraumatic stress disorder. Biol Psychiatry: Cogn Neurosci Neuroimaging 5(3):281–290. https://doi.org/10.1016/j.bpsc.2019.11.016
Yamanaka K, Waki H (2022) Conditional regulation of blood pressure in response to emotional stimuli by the central nucleus of the amygdala in rats. Front Physiol 13:820112. https://doi.org/10.3389/fphys.2022.820112
McDannald MA (2010) Contributions of the amygdala central nucleus and ventrolateral periaqueductal grey to freezing and instrumental suppression in Pavlovian fear conditioning. Behav Brain Res 211(1):111–117. https://doi.org/10.1016/j.bbr.2010.03.020
Tovote P, Esposito MS, Botta P, Chaudun F, Fadok JP, Markovic M, Wolff SBE, Ramakrishnan C, Fenno L, Deisseroth K, Herry C, Arber S, Lüthi A (2016) Midbrain circuits for defensive behaviour. Nature. https://doi.org/10.1038/nature17996
Tsang E, Orlandini C, Sureka R, Crevenna AH, Perlas E, Prankerd I, Masferrer ME, Gross CT (2023) Induction of flight via midbrain projections to the cuneiform nucleus. PLoS ONE 18(2):e0281464. https://doi.org/10.1371/journal.pone.0281464
Dampney R (2018) Emotion and the cardiovascular system: postulated role of inputs from the medial prefrontal cortex to the dorsolateral periaqueductal gray. Front Neurosci 12:343. https://doi.org/10.3389/fnins.2018.00343
Bittencourt AS, Carobrez AP, Zamprogno LP, Tufik S, Schenberg LC (2004) Organization of single components of defensive behaviors within distinct columns of periaqueductal gray matter of the rat: role of N-METHYL-d-aspartic acid glutamate receptors. Neuroscience 125(1):71–89. https://doi.org/10.1016/j.neuroscience.2004.01.026
Cowie RJ, Holstege G (1992) Dorsal mesencephalic projections to pons, medulla, and spinal cord in the cat: limbic and non-limbic components. J Comp Neurol 319(4):536–559. https://doi.org/10.1002/cne.903190406
Saha S (2005) Role of the central nucleus of the amygdala in the control of blood pressure: descending pathways to medullary cardiovascular nuclei. Clin Exp Pharmacol Physiol 32(5–6):450–456. https://doi.org/10.1111/j.1440-1681.2005.04210.x
Ulrich-Lai YM, Herman JP (2009) Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10(6):397–409. https://doi.org/10.1038/nrn2647
Zandstra TE, Notenboom RGE, Wink J, Kiès P, Vliegen HW, Egorova AD, Schalij MJ, De Ruiter MC, Jongbloed MRM (2021) Asymmetry and heterogeneity: part and parcel in cardiac autonomic innervation and function. Front Physiol 12:665298. https://doi.org/10.3389/fphys.2021.665298
Wickramasinghe SR, Patel VV (2013) Local innervation and atrial fibrillation. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.113.001596
Delaney JPA, Brodie DA (2000) Effects of short-term psychological stress on the time and frequency domains of heart-rate variability. Percept Mot Skills 91(2):515–524. https://doi.org/10.2466/pms.2000.91.2.515
de Looff PC, Cornet LJM, Embregts PJCM, Nijman HLI, Didden HCM (2018) Associations of sympathetic and parasympathetic activity in job stress and burnout: a systematic review. PLoS ONE 13(10):e0205741. https://doi.org/10.1371/journal.pone.0205741
Ritz T, Schulz SM, Rosenfield D, Wright RJ, Bosquet Enlow M (2020) Cardiac sympathetic activation and parasympathetic withdrawal during psychosocial stress exposure in six-month old infants. Psychophysiology 57(12):e13673. https://doi.org/10.1111/psyp.13673
Schuurmans AAT, Nijhof KS, Cima M, Scholte R, Popma A, Otten R (2021) Alterations of autonomic nervous system and HPA axis basal activity and reactivity to acute stress: a comparison of traumatized adolescents and healthy controls. Stress 24(6):876–887. https://doi.org/10.1080/10253890.2021.1900108
Head GA, Burke SL (2004) Sympathetic responses to stress and rilmenidine in 2K1C rabbits. Hypertension 43(3):636–642. https://doi.org/10.1161/01.HYP.0000116301.02975.aa
Furlong TM, McDowall LM, Horiuchi J, Polson JW, Dampney RAL (2014) The effect of air puff stress on c-Fos expression in rat hypothalamus and brainstem: central circuitry mediating sympathoexcitation and baroreflex resetting. Eur J Neurosci 39(9):1429–1438. https://doi.org/10.1111/ejn.12521
Kondo N, Yoshimoto M, Ikegame S, Miki K (2021) Differential shifts in baroreflex control of renal and lumbar sympathetic nerve activity induced by freezing behaviour in rats. Exp Physiol 106(10):2060–2069. https://doi.org/10.1113/EP089742
López-González MV, Díaz-Casares A, González-García M, Peinado-Aragonés CA, Barbancho MA, Carrillo de Albornoz M, Dawid-Milner MS (2018) Glutamate receptors of the A5 region modulate cardiovascular responses evoked from the dorsomedial hypothalamic nucleus and perifornical area. J Physiol Biochem 74(2):325–334. https://doi.org/10.1007/s13105-018-0623-3
Gao H-R, Zhuang Q-X, Li B, Li H-Z, Chen Z-P, Wang J-J, Zhu J-N (2016) Corticotropin releasing factor excites neurons of posterior hypothalamic nucleus to produce tachycardia in rats. Sci Rep 6:20206. https://doi.org/10.1038/srep20206
Barretto-de-Souza L, Benini R, Reis-Silva LL, Crestani CC (2021) Corticotropin-releasing factor neurotransmission in the lateral hypothalamus modulates the tachycardiac response during acute emotional stress in rats. Brain Res Bull 166:102–109. https://doi.org/10.1016/j.brainresbull.2020.11.010
Lambert E, Dawood T, Schlaich M, Straznicky N, Esler M, Lambert G (2008) Single-unit sympathetic discharge pattern in pathological conditions associated with elevated cardiovascular risk. Clin Exp Pharmacol Physiol 35(4):503–507. https://doi.org/10.1111/j.1440-1681.2008.04905.x
Bremner JD, Campanella C, Khan Z, Shah M, Hammadah M, Wilmot K, Mheid IA, Lima BB, Garcia EV, Nye J, Ward L, Kutner MH, Raggi P, Pearce BD, Shah A, Quyyumi A, Vaccarino V (2018) Brain correlates of mental stress-induced myocardial ischemia. Psychosom Med 80(6):515–525. https://doi.org/10.1097/PSY.0000000000000597
Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, Truong QA, Solomon CJ, Calcagno C, Mani V, Tang CY, Mulder WJ, Murrough JW, Hoffmann U, Nahrendorf M, Shin LM, Fayad ZA, Pitman RK (2017) Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. The Lancet 389(10071):834–845. https://doi.org/10.1016/S0140-6736(16)31714-7
Baron R, Jänig W (1988) Sympathetic and afferent neurons projecting in the splenic nerve of the cat. Neurosci Lett 94(1):109–113. https://doi.org/10.1016/0304-3940(88)90279-0
Bellinger DL, Lorton D (2018) Sympathetic Nerve hyperactivity in the spleen: causal for nonpathogenic-driven chronic immune-mediated inflammatory diseases (IMIDs)? Int J Mol Sci 19(4):1188. https://doi.org/10.3390/ijms19041188
Polimanti R, Wendt FR, Pathak GA, Tylee DS, Tcheandjieu C, Hilliard AT, Levey DF, Adhikari K, Gaziano JM, O’Donnell CJ, Assimes TL, Stein MB, Gelernter J (2022) Understanding the comorbidity between posttraumatic stress severity and coronary artery disease using genome-wide information and electronic health records. Mol Psychiatry. https://doi.org/10.1038/s41380-022-01735-z
Gianaros PJ, Hariri AR, Sheu LK, Muldoon MF, Sutton-Tyrrell K, Manuck SB (2009) Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biol Psychiat 65(11):943–950. https://doi.org/10.1016/j.biopsych.2008.10.007
Ghiadoni L, Donald AE, Cropley M, Mullen MJ, Oakley G, Taylor M, O’Connor G, Betteridge J, Klein N, Steptoe A, Deanfield JE (2000) Mental stress induces transient endothelial dysfunction in humans. Circulation 102(20):2473–2478. https://doi.org/10.1161/01.CIR.102.20.2473
Samad Z, Boyle S, Ersboll M, Vora AN, Zhang Y, Becker RC, Williams R, Kuhn C, Ortel TL, Rogers JG, O’Connor CM, Velazquez EJ, Jiang W (2014) Sex differences in platelet reactivity and cardiovascular and psychological response to mental stress in patients with stable ischemic heart disease: insights from the Remit study. J Am Coll Cardiol 64(16):1669–1678. https://doi.org/10.1016/j.jacc.2014.04.087
Spruill TM, Butler MJ, Thomas SJ, Tajeu GS, Kalinowski J, Castañeda SF, Langford AT, Abdalla M, Blackshear C, Allison M, Ogedegbe G, Sims M, Shimbo D (2019) Association between high perceived stress over time and incident hypertension in black adults: findings from the jackson heart study. J Am Heart Assoc 8(21):e012139. https://doi.org/10.1161/JAHA.119.012139
Esler M (2010) The 2009 Carl Ludwig Lecture: pathophysiology of the human sympathetic nervous system in cardiovascular diseases: the transition from mechanisms to medical management. J Appl Physiol 108(2):227–237. https://doi.org/10.1152/japplphysiol.00832.2009
Lambert GW, Kaye DM, Lefkovits J, Jennings GL, Turner AG, Cox HS, Esler MD (1995) Increased central nervous system monoamine neurotransmitter turnover and its association with sympathetic nervous activity in treated heart failure patients. Circulation 92(7):1813–1818. https://doi.org/10.1161/01.CIR.92.7.1813
Lopez Ruiz JR, Ernst SA, Holz RW, Stuenkel EL (2022) Basal and stress-induced network activity in the adrenal medulla in vivo. Front Endocrinol 13:875865. https://doi.org/10.3389/fendo.2022.875865
Schaare HL, Blöchl M, Kumral D, Uhlig M, Lemcke L, Valk SL, Villringer A (2023) Associations between mental health, blood pressure and the development of hypertension. Nat Commun 14:1953. https://doi.org/10.1038/s41467-023-37579-6
Rodrigues B, Barboza CA, Moura EG, Ministro G, Ferreira-Melo SE, Castaño JB, Ruberti OM, De Amorim RFB, Moreno H (2021) Transcranial direct current stimulation modulates autonomic nervous system and reduces ambulatory blood pressure in hypertensives. Clin Exp Hypertens 43(4):320–327. https://doi.org/10.1080/10641963.2021.1871916
Taggart P, Critchley H, van Duijvendoden S, Lambiase PD (2016) Significance of neuro-cardiac control mechanisms governed by higher regions of the brain. Auton Neurosci 199:54–65. https://doi.org/10.1016/j.autneu.2016.08.013
Polimeni A, Spaccarotella C, Ielapi J, Esposito G, Ravera A, Martuscelli E, Ciconte V, Menichelli M, Varbella F, Imazio M, Navazio A, Sinagra G, Oberhollenzer R, Sibilio G, Cacciavillani L, Meloni L, Dominici M, Tomai F, Amico F, Indolfi C (2022) The impact of UEFA Euro 2020 football championship on takotsubo syndrome: results of a multicenter national registry. Front Cardiovasc Med 9:951882. https://doi.org/10.3389/fcvm.2022.951882
Narita K, Hoshide S, Tsoi K, Siddique S, Shin J, Chia Y, Tay JC, Teo BW, Turana Y, Chen C, Cheng H, Sogunuru GP, Wang T, Wang J, Kario K (2021) Disaster hypertension and cardiovascular events in disaster and COVID-19 pandemic. J Clin Hypertens 23(3):575–583. https://doi.org/10.1111/jch.14192
Pollatos O, Kirsch W, Schandry R (2005) On the relationship between interoceptive awareness, emotional experience, and brain processes. Cogn Brain Res 25(3):948–962. https://doi.org/10.1016/j.cogbrainres.2005.09.019
Ziegelstein RC (2007) Acute emotional stress and cardiac arrhythmias. JAMA 298(3):324–329. https://doi.org/10.1001/jama.298.3.324
Appiah D, Farias R, Helo D, Appiah L, Olokede OA, Nwabuo CC, Nair N (2021) Association of marital status with takotsubo syndrome (broken heart syndrome) among adults in the United States. World J Cardiol 13(8):340–347. https://doi.org/10.4330/wjc.v13.i8.340
Kattel S, Bhatt H, Gurung S, Karthikeyan B, Sharma UC (2021) Elevated myocardial wall stress after percutaneous coronary intervention in acute ST elevation myocardial infraction is associated with increased mortality. Echocardiography 38(8):1263–1271. https://doi.org/10.1111/echo.15131
Ferrari R (2015) Writing narrative style literature reviews. Medical Writing 24(4):230–235. https://doi.org/10.1179/2047480615Z.000000000329
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Braun, J., Patel, M., Kameneva, T. et al. Central stress pathways in the development of cardiovascular disease. Clin Auton Res 34, 99–116 (2024). https://doi.org/10.1007/s10286-023-01008-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-023-01008-x