Abstract
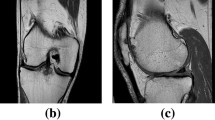
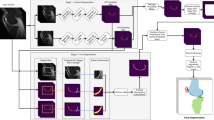
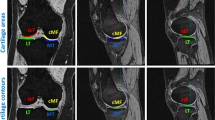
Knee osteoarthritis (OA) is a degenerative joint disease that is prevalent in advancing age. The pathology of OA disease is still unclear, and there are no effective interventions that can completely alter the OA disease process. Magnetic resonance (MR) image evaluation is sensitive for depicting early changes of knee OA, and therefore important for early clinical intervention for relieving the symptom. Automated cartilage segmentation based on MR images is a vital step in experimental longitudinal studies to follow-up the patients and prospectively define a new quantitative marker from OA progression. In this paper, we develop a deep learning–based coarse-to-fine approach for automated knee bone, cartilage, and meniscus segmentation with high computational efficiency. The proposed method is evaluated using two-fold cross-validation on 507 MR volumes (81,120 slices) with OA from the Osteoarthritis Initiative (OAI)1 dataset. The mean dice similarity coefficients (DSCs) of femoral bone (FB), tibial bone (TB), femoral cartilage (FC), and tibial cartilage (TC) separately are 99.1%, 98.2%, 90.9%, and 85.8%. The time of segmenting each patient is 12 s, which is fast enough to be used in clinical practice. Our proposed approach may provide an automated toolkit to help computer-aided quantitative analyses of OA images.







Similar content being viewed by others
References
R. C. Lawrence et al., Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II, Arthritis & Rheumatism, vol. 58, no. 1, pp. 26-35, 2008.
K. D. Brandt, Diagnosis and nonsurgical management of osteoarthritis. Professional Communications, 2010.
Y. Zhang and J. M. Jordan, Epidemiology of osteoarthritis, Clinics in geriatric medicine, vol. 26, no. 3, pp. 355-369, 2010.
C. R. Chu, A. A. Williams, C. H. Coyle, and M. E. Bowers, Early diagnosis to enable early treatment of pre-osteoarthritis, Arthritis research & therapy, vol. 14, no. 3, p. 212, 2012.
E. Yelin, S. Weinstein, and T. King, The burden of musculoskeletal diseases in the United States, in Seminars in arthritis and rheumatism, 2016, vol. 46, no. 3, p. 259.
A. Mendy, J. Park, and E. R. Vieira, Osteoarthritis and risk of mortality in the USA: a population-based cohort study, International Journal of Epidemiology, vol. 47, no. 6, pp. 1821-1829, 2018.
D. Bhatia, T. Bejarano, and M. Novo, Current interventions in the management of knee osteoarthritis, Journal of pharmacy & bioallied sciences, vol. 5, no. 1, p. 30, 2013.
M. T. Nieminen, V. Casula, M. T. Nevalainen, and S. Saarakkala, Osteoarthritis year in review 2018: imaging, Osteoarthritis and cartilage, vol. 27, no. 3, pp. 401-411, 2019.
H. Shim, S. Chang, C. Tao, J.-H. Wang, C. K. Kwoh, and K. T. Bae, Knee cartilage: efficient and reproducible segmentation on high-spatial-resolution MR images with the semiautomated graph-cut algorithm method, Radiology, vol. 251, no. 2, pp. 548-556, 2009.
Y. Du, R. Almajalid, J. Shan, and M. Zhang, A novel method to predict knee osteoarthritis progression on MRI using machine learning methods, IEEE transactions on nanobioscience, vol. 17, no. 3, pp. 228-236, 2018.
J. Jaremko, R. Cheng, R. Lambert, A. Habib, and J. Ronsky, Reliability of an efficient MRI-based method for estimation of knee cartilage volume using surface registration, Osteoarthritis and cartilage, vol. 14, no. 9, pp. 914-922, 2006.
F. Eckstein and W. Wirth, Quantitative cartilage imaging in knee osteoarthritis, Arthritis, vol. 2011, 2011.
J. Fripp, S. Crozier, S. K. Warfield, and S. Ourselin, Automatic segmentation and quantitative analysis of the articular cartilages from magnetic resonance images of the knee, IEEE transactions on medical imaging, vol. 29, no. 1, pp. 55-64, 2009.
Z. Javaid, M. G. Boocock, P. J. McNair, and C. P. Unsworth, Contour interpolated radial basis functions with spline boundary correction for fast 3D reconstruction of the human articular cartilage from MR images, Medical Physics, vol. 43, no. 3, pp. 1187-1199, 2016.
J. G. Lee, S. Gumus, C. H. Moon, C. K. Kwoh, and K. T. Bae, Fully automated segmentation of cartilage from the MR images of knee using a multi‐atlas and local structural analysis method, Medical physics, vol. 41, no. 9, p. 092303, 2014.
Y. Yin, X. Zhang, R. Williams, X. Wu, D. D. Anderson, and M. Sonka, LOGISMOS—layered optimal graph image segmentation of multiple objects and surfaces: cartilage segmentation in the knee joint, IEEE transactions on medical imaging, vol. 29, no. 12, pp. 2023-2037, 2010.
P. M. Cashman, R. I. Kitney, M. A. Gariba, and M. E. Carter, Automated techniques for visualization and mapping of articular cartilage in MR images of the osteoarthritic knee: a base technique for the assessment of microdamage and submicro damage, IEEE transactions on nanobioscience, vol. 99, no. 1, pp. 42-51, 2002.
H. Z. Tameem and U. S. Sinha, Automated image processing and analysis of cartilage MRI: enabling technology for data mining applied to osteoarthritis, in AIP conference proceedings, 2007, vol. 953, no. 1, pp. 262–276: American Institute of Physics.
G. Vincent, C. Wolstenholme, I. Scott, and M. Bowes, Fully automatic segmentation of the knee joint using active appearance models, Medical Image Analysis for the Clinic: A Grand Challenge, vol. 1, p. 224, 2010.
V. Pedoia, S. Majumdar, and T. M. Link, Segmentation of joint and musculoskeletal tissue in the study of arthritis, Magnetic Resonance Materials in Physics, Biology and Medicine, vol. 29, no. 2, pp. 207-221, 2016.
M. Zhang et al., Development of a rapid knee cartilage damage quantification method using magnetic resonance images, BMC musculoskeletal disorders, vol. 15, no. 1, p. 264, 2014.
M. Zhang, J. B. Driban, L. L. Price, G. H. Lo, E. Miller, and T. E. McAlindon, Development of a rapid cartilage damage quantification method for the lateral tibiofemoral compartment using magnetic resonance images: data from the osteoarthritis initiative, BioMed research international, vol. 2015, 2015.
F. Ambellan, A. Tack, M. Ehlke, and S. Zachow, Automated segmentation of knee bone and cartilage combining statistical shape knowledge and convolutional neural networks: Data from the Osteoarthritis Initiative, Medical image analysis, vol. 52, pp. 109-118, 2019.
Z. Zhou, M. M. R. Siddiquee, N. Tajbakhsh, and J. Liang, Unet++: Redesigning skip connections to exploit multiscale features in image segmentation, IEEE transactions on medical imaging, vol. 39, no. 6, pp. 1856-1867, 2019.
O. Ronneberger, P. Fischer, and T. Brox, U-net: Convolutional networks for biomedical image segmentation, in International Conference on Medical image computing and computer-assisted intervention, 2015, pp. 234–241: Springer.
B. Park, H. Park, S. M. Lee, J. B. Seo, and N. Kim, Lung segmentation on HRCT and volumetric CT for diffuse interstitial lung disease using deep convolutional neural networks, Journal of Digital Imaging, vol. 32, no. 6, pp. 1019-1026, 2019.
G. Singadkar, A. Mahajan, M. Thakur, and S. Talbar, Deep deconvolutional residual network based automatic lung nodule segmentation, Journal of Digital Imaging, pp. 1–7, 2020.
H. Seim, D. Kainmueller, H. Lamecker, M. Bindernagel, J. Malinowski, and S. Zachow, Model-based auto-segmentation of knee bones and cartilage in MRI data, Proc. Medical Image Analysis for the Clinic: A Grand Challenge. Bejing, China, pp. 215–223, 2010.
M. H. Hesamian, W. Jia, X. He, and P. Kennedy, Deep learning techniques for medical image segmentation: achievements and challenges, Journal of digital imaging, vol. 32, no. 4, pp. 582-596, 2019.
J. Long, E. Shelhamer, and T. Darrell, Fully convolutional networks for semantic segmentation, in Proceedings of the IEEE conference on computer vision and pattern recognition, 2015, pp. 3431–3440.
K. Simonyan and A. Zisserman, Very deep convolutional networks for large-scale image recognition, arXiv preprint arXiv:1409.1556, 2014.
K. He, X. Zhang, S. Ren, and J. Sun, Deep residual learning for image recognition, in Proceedings of the IEEE conference on computer vision and pattern recognition, 2016, pp. 770–778.
D. P. Kingma and J. Ba, Adam: A method for stochastic optimization, arXiv preprint arXiv:1412.6980, 2014.
T.-Y. Lin, P. Goyal, R. Girshick, K. He, and P. Dollár, Focal loss for dense object detection, in Proceedings of the IEEE international conference on computer vision, 2017, pp. 2980–2988.
J. M. Jordan et al., Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project, The Journal of rheumatology, vol. 34, no. 1, pp. 172-180, 2007.
O. Oktay et al., Attention u-net: learning where to look for the pancreas, arXiv preprint arXiv:1804.03999, 2018.
D. Dreizin, Y. Zhou, Y. Zhang, N. Tirada, and A. L. Yuille, Performance of a deep learning algorithm for automated segmentation and quantification of traumatic pelvic hematomas on CT, Journal of digital imaging, vol. 33, no. 1, pp. 243-251, 2020.
Q. Yu, L. Xie, Y. Wang, Y. Zhou, E. K. Fishman, and A. L. Yuille, Recurrent saliency transformation network: Incorporating multi-stage visual cues for small organ segmentation, in Proceedings of the IEEE conference on computer vision and pattern recognition, 2018, pp. 8280–8289.
Acknowledgements
We thank Zuse Institute Berlin for kindly providing annotations.
Funding
This work was supported in part by the National Institutes of Health (NIH) under Grant 1R01DE027027-02 and Grant 1U01 AR069395-03 (XZ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deng, Y., You, L., Wang, Y. et al. A Coarse-to-Fine Framework for Automated Knee Bone and Cartilage Segmentation Data from the Osteoarthritis Initiative. J Digit Imaging 34, 833–840 (2021). https://doi.org/10.1007/s10278-021-00464-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10278-021-00464-z